
Chronic granulomatous disease (CGD), also known as Bridges–Good syndrome, chronic granulomatous disorder, and Quie syndrome, is a diverse group of hereditary diseases in which certain cells of the immune system have difficulty forming the reactive oxygen compounds used to kill certain ingested pathogens. This leads to the formation of granulomas in many organs. CGD affects about 1 in 200,000 people in the United States, with about 20 new cases diagnosed each year.

X-linked agammaglobulinemia (XLA) is a rare genetic disorder discovered in 1952 that affects the body's ability to fight infection. As the form of agammaglobulinemia that is X-linked, it is much more common in males. In people with XLA, the white blood cell formation process does not generate mature B cells, which manifests as a complete or near-complete lack of proteins called gamma globulins, including antibodies, in their bloodstream. B cells are part of the immune system and normally manufacture antibodies, which defend the body from infections by sustaining a humoral immunity response. Patients with untreated XLA are prone to develop serious and even fatal infections. A mutation occurs at the Bruton's tyrosine kinase (Btk) gene that leads to a severe block in B cell development and a reduced immunoglobulin production in the serum. Btk is particularly responsible for mediating B cell development and maturation through a signaling effect on the B cell receptor BCR. Patients typically present in early childhood with recurrent infections, in particular with extracellular, encapsulated bacteria. XLA is deemed to have a relatively low incidence of disease, with an occurrence rate of approximately 1 in 200,000 live births and a frequency of about 1 in 100,000 male newborns. It has no ethnic predisposition. XLA is treated by infusion of human antibody. Treatment with pooled gamma globulin cannot restore a functional population of B cells, but it is sufficient to reduce the severity and number of infections due to the passive immunity granted by the exogenous antibodies.
Hypogammaglobulinemia is a problem with the immune system in which not enough gamma globulins are produced in the blood. This results in a lower antibody count, which impairs the immune system, increasing risk of infection. Hypogammaglobulinemia may result from a variety of primary genetic immune system defects, such as common variable immunodeficiency, or it may be caused by secondary effects such as medication, blood cancer, or poor nutrition, or loss of gamma globulins in urine, as in nonselective glomerular proteinuria. Patients with hypogammaglobulinemia have reduced immune function; important considerations include avoiding use of live vaccines, and take precautionary measures when traveling to regions with endemic disease or poor sanitation such as receiving immunizations, taking antibiotics abroad, drinking only safe or boiled water, arranging appropriate medical cover in advance of travel, and ensuring continuation of any immunoglobulin infusions needed.
Hyperimmunoglobulinemia E syndrome (HIES), of which the autosomal dominant form is called Job's syndrome or Buckley syndrome, is a heterogeneous group of immune disorders. Job's is also very rare at about 300 cases currently in the literature.

Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase.
Vici syndrome, also called immunodeficiency with cleft lip/palate, cataract, hypopigmentation and absent corpus callosum, is a rare autosomal recessive congenital disorder characterized by albinism, agenesis of the corpus callosum, cataracts, cardiomyopathy, severe psychomotor retardation, seizures, immunodeficiency and recurrent severe infections. To date, about 50 cases have been reported.

RhoGEF domain describes two distinct structural domains with guanine nucleotide exchange factor (GEF) activity to regulate small GTPases in the Rho family. Rho small GTPases are inactive when bound to GDP but active when bound to GTP; RhoGEF domains in proteins are able to promote GDP release and GTP binding to activate specific Rho family members, including RhoA, Rac1 and Cdc42.

Dock180, also known as DOCK1, is a large protein involved in intracellular signalling networks. It is the mammalian ortholog of the C. elegans protein CED-5 and belongs to the DOCK family of Guanine nucleotide exchange factors (GEFs).

Dock2, also known as DOCK2, is a large protein involved in intracellular signalling networks. It is a member of the DOCK-A subfamily of the DOCK family of guanine nucleotide exchange factors (GEFs) which function as activators of small G proteins. Dock2 specifically activates isoforms of the small G protein Rac.

Dock7, also known as Zir2, is a large protein involved in intracellular signalling networks. It is a member of the DOCK-C subfamily of the DOCK family of guanine nucleotide exchange factors (GEFs) which function as activators of small G proteins. Dock7 activates isoforms of the small G protein Rac.

Dedicator of cytokinesis protein 10 (Dock10), also known as Zizimin3, is a large protein involved in intracellular signalling networks that in humans is encoded by the DOCK10 gene. It is a member of the DOCK-D subfamily of the DOCK family of guanine nucleotide exchange factors, which function as activators of small G proteins.

Dock4, also known as DOCK4, is a large protein involved in intracellular signalling networks. It is a member of the DOCK-B subfamily of the DOCK family of guanine nucleotide exchange factors (GEFs) which function as activators of small G proteins. Dock4 activates the small G proteins Rac and Rap1.

Dock3, also known as MOCA and PBP, is a large protein involved in intracellular signalling networks. It is a member of the DOCK-B subfamily of the DOCK family of guanine nucleotide exchange factors (GEFs) which function as activators of small G proteins. Dock3 specifically activates the small G protein Rac.
Dock9, also known as Zizimin1, is a large protein involved in intracellular signalling networks. It is a member of the DOCK-D subfamily of the DOCK family of guanine nucleotide exchange factors that function as activators of small G proteins. Dock9 activates the small G protein Cdc42.
DHR1, also known as CZH1 or Docker1, is a protein domain of approximately 200–250 amino acids that is present in the DOCK family of signalling proteins. This domain binds phospholipids and so may assist in recruitment to cellular membranes. There is evidence that this domain may also mediate protein–protein interactions.
DHR2, also known as CZH2 or Docker2, is a protein domain of approximately 450-550 amino acids that is present in the DOCK family of proteins. This domain functions as a guanine nucleotide exchange factor (GEF) domain for small G proteins of the Rho family. DHR2 domains bear no significant similarity to the well described DH domain present in other RhoGEFs such as Vav, P-Rex and TRIO. Indeed, the most divergent mammalian DHR2 domains share only 16-17% sequence similarity.

Dock5, also known as DOCK5, is a large protein involved in intracellular signalling networks. It is a member of the DOCK-A subfamily of the DOCK family of guanine nucleotide exchange factors (GEFs) which function as activators of small G proteins. Dock5 is predicted to activate the small G protein Rac.

Dock6, also known as Zir1 is a large protein involved in intracellular signalling networks. It is a member of the DOCK-C subfamily of the DOCK family of guanine nucleotide exchange factors which function as activators of small G proteins.

Dock11, also known as Zizimin2, is a large protein involved in intracellular signalling networks. It is a member of the DOCK-D subfamily of the DOCK family of guanine nucleotide exchange factors (GEFs) which function as activators of small G proteins. Dock11 activates the small G protein Cdc42.
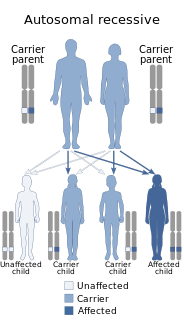
DOCK8 deficiency, also called DOCK8 immunodeficiency syndrome, is the autosomal recessive form of hyperimmunoglobulin E syndrome, a genetic disorder characterized by elevated immunoglobulin E levels, eosinophilia, and recurrent infections with staphylococcus and viruses. It is caused by a mutation in the DOCK8 gene.


















