
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidity affects rate of evaporation of water. When the molecules of the liquid collide, they transfer energy to each other based on how they collide. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling.

Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's thermodynamic tendency to evaporate. It relates to the balance of particles escaping from the liquid in equilibrium with those in a coexisting vapor phase. A substance with a high vapor pressure at normal temperatures is often referred to as volatile. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the attractive interactions between liquid molecules become less significant in comparison to the entropy of those molecules in the gas phase, increasing the vapor pressure. Thus, liquids with strong intermolecular interactions are likely to have smaller vapor pressures, with the reverse true for weaker interactions.
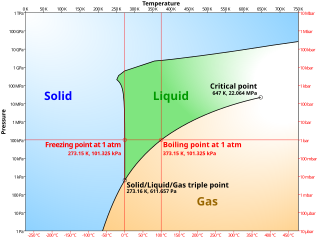
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases occur and coexist at equilibrium.

In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
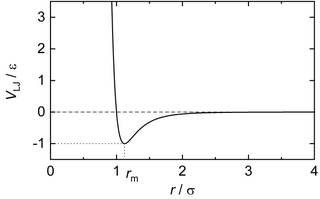
In computational chemistry, molecular physics, and physical chemistry, the Lennard-Jones potential is an intermolecular pair potential. Out of all the intermolecular potentials, the Lennard-Jones potential is probably the one that has been the most extensively studied. It is considered an archetype model for simple yet realistic intermolecular interactions. The Lennard-Jones potential is often used as a building block in molecular models for more complex substances. Many studies of the idealized "Lennard-Jones substance" use the potential to understand the physical nature of matter.

Psychrometrics is the field of engineering concerned with the physical and thermodynamic properties of gas-vapor mixtures.
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed directly in terms of simple concentrations or partial pressures of the substances present e.g. Raoult's law. Deviations from ideality are accommodated by modifying the concentration by an activity coefficient. Analogously, expressions involving gases can be adjusted for non-ideality by scaling partial pressures by a fugacity coefficient.
This page provides supplementary data to the article properties of water.
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase.
Jürgen Gmehling is a retired German professor of technical and industrial chemistry at the Carl von Ossietzky University of Oldenburg.

In thermodynamics, vapor quality is the mass fraction in a saturated mixture that is vapor; in other words, saturated vapor has a "quality" of 100%, and saturated liquid has a "quality" of 0%. Vapor quality is an intensive property which can be used in conjunction with other independent intensive properties to specify the thermodynamic state of the working fluid of a thermodynamic system. It has no meaning for substances which are not saturated mixtures . Vapor quality is an important quantity during the adiabatic expansion step in various thermodynamic cycles. Working fluids can be classified by using the appearance of droplets in the vapor during the expansion step.

In statistical thermodynamics, the UNIFAC method is a semi-empirical system for the prediction of non-electrolyte activity in non-ideal mixtures. UNIFAC uses the functional groups present on the molecules that make up the liquid mixture to calculate activity coefficients. By using interactions for each of the functional groups present on the molecules, as well as some binary interaction coefficients, the activity of each of the solutions can be calculated. This information can be used to obtain information on liquid equilibria, which is useful in many thermodynamic calculations, such as chemical reactor design, and distillation calculations.

The non-random two-liquid model is an activity coefficient model introduced by Renon and Prausnitz in 1968 that correlates the activity coefficients of a compound with its mole fractions in the liquid phase concerned. It is frequently applied in the field of chemical engineering to calculate phase equilibria. The concept of NRTL is based on the hypothesis of Wilson, who stated that the local concentration around a molecule in most mixtures is different from the bulk concentration. This difference is due to a difference between the interaction energy of the central molecule with the molecules of its own kind and that with the molecules of the other kind . The energy difference also introduces a non-randomness at the local molecular level. The NRTL model belongs to the so-called local-composition models. Other models of this type are the Wilson model, the UNIQUAC model, and the group contribution model UNIFAC. These local-composition models are not thermodynamically consistent for a one-fluid model for a real mixture due to the assumption that the local composition around molecule i is independent of the local composition around molecule j. This assumption is not true, as was shown by Flemr in 1976. However, they are consistent if a hypothetical two-liquid model is used. Models, which have consistency between bulk and the local molecular concentrations around different types of molecules are COSMO-RS, and COSMOSPACE.

In statistical thermodynamics, UNIQUAC is an activity coefficient model used in description of phase equilibria. The model is a so-called lattice model and has been derived from a first order approximation of interacting molecule surfaces. The model is, however, not fully thermodynamically consistent due to its two-liquid mixture approach. In this approach the local concentration around one central molecule is assumed to be independent from the local composition around another type of molecule.
A group-contribution method in chemistry is a technique to estimate and predict thermodynamic and other properties from molecular structures.
The UNIFAC Consortium was founded at the Carl von Ossietzky University of Oldenburg at the chair of industrial chemistry of Prof. Gmehling to invite private companies to support the further development of the group-contribution methods UNIFAC and its successor modified UNIFAC (Dortmund). Both models are used for the prediction of thermodynamic properties, especially the estimation of phase equilibria.
PSRK is an estimation method for the calculation of phase equilibria of mixtures of chemical components. The original goal for the development of this method was to enable the estimation of properties of mixtures containing supercritical components. This class of substances cannot be predicted with established models, for example UNIFAC.
The Margules activity model is a simple thermodynamic model for the excess Gibbs free energy of a liquid mixture introduced in 1895 by Max Margules. After Lewis had introduced the concept of the activity coefficient, the model could be used to derive an expression for the activity coefficients of a compound i in a liquid, a measure for the deviation from ideal solubility, also known as Raoult's law.
The Van Laar equation is a thermodynamic activity model, which was developed by Johannes van Laar in 1910-1913, to describe phase equilibria of liquid mixtures. The equation was derived from the Van der Waals equation. The original van der Waals parameters didn't give good description of vapor-liquid equilibria of phases, which forced the user to fit the parameters to experimental results. Because of this, the model lost the connection to molecular properties, and therefore it has to be regarded as an empirical model to correlate experimental results.
VTPR is an estimation method for the calculation of phase equilibria of mixtures of chemical components. The original goal for the development of this method was to enable the estimation of properties of mixtures which contain supercritical components. These class of substances couldn't be predicted with established models like UNIFAC.














