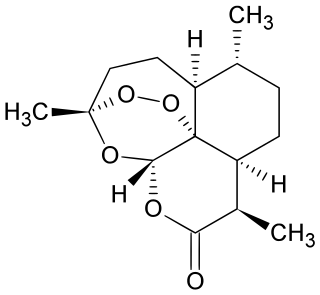
Artemisinin and its semisynthetic derivatives are a group of drugs used in the treatment of malaria due to Plasmodium falciparum. It was discovered in 1972 by Tu Youyou, who shared the 2015 Nobel Prize in Physiology or Medicine for her discovery. Artemisinin-based combination therapies (ACTs) are now standard treatment worldwide for P. falciparum malaria as well as malaria due to other species of Plasmodium. Artemisinin is extracted from the plant Artemisia annua an herb employed in Chinese traditional medicine. A precursor compound can be produced using a genetically engineered yeast, which is much more efficient than using the plant.
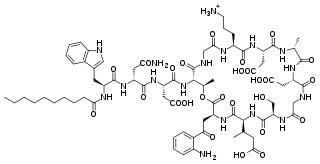
Daptomycin, sold under the brand name Cubicin among others, is a lipopeptide antibiotic used in the treatment of systemic and life-threatening infections caused by Gram-positive organisms.

Prodigiosin is the red dye produced by many strains of the bacterium Serratia marcescens, as well as other Gram-negative, gamma proteobacteria such as Vibrio psychroerythrus and Hahella chejuensis. It is responsible for the pink tint occasionally found in grime that accumulates on porcelain surfaces such as bathtubs, sinks, and toilet bowls. It is in the prodiginine family of compounds which are produced in some Gram-negative gamma proteobacteria, as well as select Gram-positive Actinobacteria. The name prodigiosin is derived from prodigious.
An apicoplast is a derived non-photosynthetic plastid found in most Apicomplexa, including Toxoplasma gondii, and Plasmodium falciparum and other Plasmodium spp., but not in others such as Cryptosporidium. It originated from algae through secondary endosymbiosis; there is debate as to whether this was a green or red alga. The apicoplast is surrounded by four membranes within the outermost part of the endomembrane system. The apicoplast hosts important metabolic pathways like fatty acid synthesis, isoprenoid precursor synthesis and parts of the heme biosynthetic pathway.

Cycloguanil is a dihydrofolate reductase inhibitor, and is a metabolite of the antimalarial drug proguanil; its formation in vivo has been thought to be primarily responsible for the antimalarial activity of proguanil. However, more recent work has indicated that, while proguanil is synergistic with the drug atovaquone, cycloguanil is in fact antagonistic to the effects of atovaquone, suggesting that, unlike cycloguanil, proguanil may have an alternative mechanism of antimalarial action besides dihydrofolate reductase inhibition.

Toll-like receptor 6 is a protein that in humans is encoded by the TLR6 gene. TLR6 is a transmembrane protein, member of toll-like receptor family, which belongs to the pattern recognition receptor (PRR) family. TLR6 acts in a heterodimer form with toll-like receptor 2 (TLR2). Its ligands include multiple diacyl lipopeptides derived from gram-positive bacteria and mycoplasma and several fungal cell wall saccharides. After dimerizing with TLR2, the NF-κB intracellular signalling pathway is activated, leading to a pro-inflammatory cytokine production and activation of innate immune response. TLR6 has also been designated as CD286.
A lipopeptide is a molecule consisting of a lipid connected to a peptide. They are able to self-assemble into different structures. Many bacteria produce these molecules as a part of their metabolism, especially those of the genus Bacillus, Pseudomonas and Streptomyces. Certain lipopeptides are used as antibiotics. Due to the structural and molecular properties such as the fatty acid chain, it poses the effect of weakening the cell function or destroying the cell. Other lipopeptides are toll-like receptor agonists. Certain lipopeptides can have strong antifungal and hemolytic activities. It has been demonstrated that their activity is generally linked to interactions with the plasma membrane, and sterol components of the plasma membrane could play a major role in this interaction. It is a general trend that adding a lipid group of a certain length to a lipopeptide will increase its bactericidal activity. Lipopeptides with a higher amount of carbon atoms, for example 14 or 16, in its lipid tail will typically have antibacterial activity as well as anti-fungal activity. Therefore, an increase in the alkyl chain can make lipopeptides soluble in water. As well, it opens the cell membrane of the bacteria, so antimicrobial activity can take place.
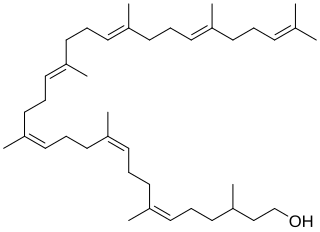
Bactoprenol also known as dolichol-11 and C55-isoprenyl alcohol (C55-OH) is a lipid first identified in certain species of lactobacilli. It is a hydrophobic alcohol that plays a key role in the growth of cell walls (peptidoglycan) in Gram-positive bacteria.
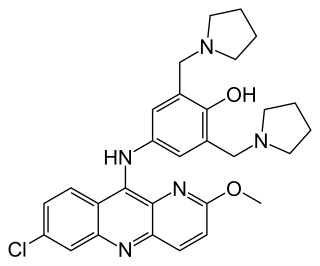
Pyronaridine is an antimalarial drug. It was first made in 1970 and has been in clinical use in China since the 1980s.
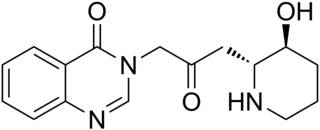
Febrifugine is a quinazolinone alkaloid first isolated from the Chinese herb Dichroa febrifuga, but also found in the garden plant Hydrangea. Laboratory synthesis of febrifugine determined that the originally reported stereochemistry was incorrect.

Lyngbya majuscula is a species of filamentous cyanobacteria in the genus Lyngbya. It is named after the Dane Hans Christian Lyngbye.

Antillatoxin (ATX) is a potent lipopeptide neurotoxin produced by the marine cyanobacterium Lyngbya majuscula. ATX activates voltage-gated sodium channels, which can cause cell depolarisation, NMDA-receptor overactivity, excess calcium influx and neuronal necrosis.
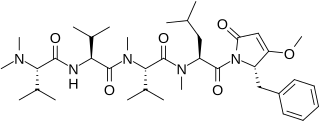
Caldoramide is a pentapeptide isolated from the cyanobacteria Caldora penicillata. It has cytotoxic effects on cancer cells and has been the subject of extensive oncological research. It is structurally analogous to belamide A and dolastatin 15. Its appearance is that of a powdery, white, substance.

The arylomycins are a class of antibiotics initially isolated from a soil sample obtained in Cape Coast, Ghana. In this initial isolation, two families of closely related arylomycins, A and B, were identified. The family of glycosylated arylomycin C lipopeptides were subsequently isolated from a Streptomyces culture in a screen for inhibitors of bacterial signal peptidase. The initially isolated arylomycins have a limited spectrum of activity against Gram-positive bacteria, including Staphylococcus aureus and Streptococcus pneumoniae. The only activity against Gram-negative bacteria was seen in strains with a compromised outer membrane.

The prodiginines are a family of red tripyrrole dyestuffs produced by Gammaproteobacteria as well as some Actinomycetota. The group is named after prodigiosin (prodiginine) and is biosynthesized through a common set of enzymes. They are interesting due to their history and their varied biological activity.
A proteolipid is a protein covalently linked to lipid molecules, which can be fatty acids, isoprenoids or sterols. The process of such a linkage is known as protein lipidation, and falls into the wider category of acylation and post-translational modification. Proteolipids are abundant in brain tissue, and are also present in many other animal and plant tissues. They include ghrelin, a peptide hormone associated with feeding. Many proteolipids are composed of proteins covalenently bound to fatty acid chains, often granting them an interface for interacting with biological membranes. They are not to be confused with lipoproteins, a kind of spherical assembly made up of many molecules of lipids and some apolipoproteins.
Laucysteinamide A (LcA) is a marine natural product isolated from a cyanobacterium, Caldora penicillata.
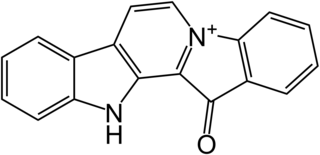
Fascaplysin is a marine alkaloid based on 12H-pyrido[1–2-a:3,4-b′]diindole ring system. It was first isolated as a red pigment from the marine sponge Fascaplysinopsis reticulata that was collected in the South Pacific near Fiji in 1988. Fascaplysin possesses a broad range of in vitro biological activities including analgesic, antimicrobial, antifungal, antiviral, antimalarial, anti-angiogenic, and antiproliferative activity against numerous cancer cell lines.

Ferroquine is a synthetic compound related to chloroquine which acts as an antimalarial, and shows good activity against chloroquine-resistant strains. It contains an organometallic ferrocene ring which is unusual in pharmaceuticals, and while it was first reported in 1997, it has progressed slowly through clinical trials, with results from Phase II trials showing reasonable safety and efficacy, and further trials ongoing.

Gallinamide A is potent and selective inhibitor of the human cysteine protease Cathepsin L1 that was first used as a moderate antimalarial agent. Gallinamide A is produced by marine cyanobacteria from Schizothrix species and Symploca sp. which have also shown to have possible anticancer agent, infectious diseases like leishmaniasis, trypanosomiasis and possible uses in Alzheimer's disease, among others.
















