ERO1-like protein alpha is a protein that in humans is encoded by the ERO1L gene. [5] [6]
ERO1-like protein alpha is a protein that in humans is encoded by the ERO1L gene. [5] [6]
ERO1L has been shown to interact with TXNDC4 [7] and P4HB. [7] [8]

Protein disulfide isomerase, or PDI, is an enzyme in the endoplasmic reticulum (ER) in eukaryotes and the periplasm of bacteria that catalyzes the formation and breakage of disulfide bonds between cysteine residues within proteins as they fold. This allows proteins to quickly find the correct arrangement of disulfide bonds in their fully folded state, and therefore the enzyme acts to catalyze protein folding.

Calnexin (CNX) is a 67kDa integral protein of the endoplasmic reticulum (ER). It consists of a large N-terminal calcium-binding lumenal domain, a single transmembrane helix and a short, acidic cytoplasmic tail. In humans, calnexin is encoded by the gene CANX.

ER oxidoreductin 1 (Ero1) is an oxidoreductase enzyme that catalyses the formation and isomerization of protein disulfide bonds in the endoplasmic reticulum (ER) of eukaryotes. ER Oxidoreductin 1 (Ero1) is a conserved, luminal, glycoprotein that is tightly associated with the ER membrane, and is essential for the oxidation of protein dithiols. Since disulfide bond formation is an oxidative process, the major pathway of its catalysis has evolved to utilise oxidoreductases, which become reduced during the thiol-disulfide exchange reactions that oxidise the cysteine thiol groups of nascent polypeptides. Ero1 is required for the introduction of oxidising equivalents into the ER and their direct transfer to protein disulfide isomerase (PDI), thereby ensuring the correct folding and assembly of proteins that contain disulfide bonds in their native state.
The unfolded protein response (UPR) is a cellular stress response related to the endoplasmic reticulum (ER) stress. It has been found to be conserved between mammalian species, as well as yeast and worm organisms.

Protein disulfide-isomerase A3 (PDIA3), also known as glucose-regulated protein, 58-kD (GRP58), is an isomerase enzyme encoded by the autosomal gene PDIA3 in humans. This protein localizes to the endoplasmic reticulum (ER) and interacts with lectin chaperones calreticulin and calnexin (CNX) to modulate folding of newly synthesized glycoproteins. It is thought that complexes of lectins and this protein mediate protein folding by promoting formation of disulfide bonds in their glycoprotein substrates.

Protein disulfide-isomerase, also known as the beta-subunit of prolyl 4-hydroxylase (P4HB), is an enzyme that in humans encoded by the P4HB gene. The human P4HB gene is localized in chromosome 17q25. Unlike other prolyl 4-hydroxylase family proteins, this protein is multifunctional and acts as an oxidoreductase for disulfide formation, breakage, and isomerization. The activity of P4HB is tightly regulated. Both dimer dissociation and substrate binding are likely to enhance its enzymatic activity during the catalysis process.

E3 ubiquitin-protein ligase synoviolin is an enzyme that in humans is encoded by the SYVN1 gene.

KDEL (Lys-Asp-Glu-Leu) endoplasmic reticulum protein retention receptor 1, also known as KDELR1, is a protein which in humans is encoded by the KDELR1 gene.
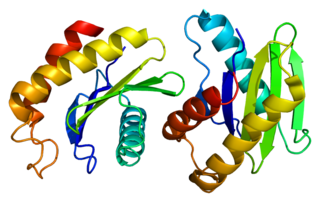
Vesicle-trafficking protein SEC22b is a protein that in humans is encoded by the SEC22B gene.

Endoplasmic reticulum resident protein 44 (ERp44) also known as thioredoxin domain-containing protein 4 (TXNDC4) is a protein that in humans is encoded by the ERP44 gene.

Dolichol-phosphate mannosyltransferase is an enzyme that in humans is encoded by the DPM1 gene.

Phosphatidylinositol N-acetylglucosaminyltransferase subunit C is an enzyme that in humans is encoded by the PIGC gene.

ERO1-like protein beta is a protein that in humans is encoded by the ERO1LB gene.

DnaJ homolog subfamily C member 10 is a protein that in humans is encoded by the DNAJC10 gene.

GPI transamidase component PIG-S is an enzyme that in humans is encoded by the PIGS gene. This gene encodes a protein that is involved in GPI-anchor biosynthesis.

Phosphatidylinositol N-acetylglucosaminyltransferase subunit H is an enzyme that in humans is encoded by the PIGH gene. The PIGH gene is located on the reverse strand of chromosome 14 in humans, and is neighbored by TMEM229B.

dolichyl-phosphate mannosyltransferase polypeptide 3, also known as DPM3, is a human gene.

Dolichol phosphate-mannose biosynthesis regulatory protein is a protein that in humans is encoded by the DPM2 gene.
A FFAT motif is a protein sequence motif of six defined amino acids plus neighbouring residues that binds to proteins in the VAP protein family.

Protein disulfide isomerase family A member 2 is a protein that in humans is encoded by the PDIA2 gene.