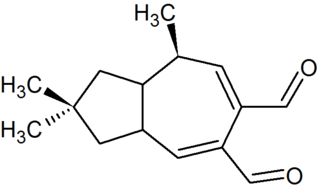Naphthalene is an organic compound with formula C
10H
8. It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is best known as the main ingredient of traditional mothballs.

In chemistry, aromaticity is a property of cyclic (ring-shaped), planar (flat) structures with pi bonds in resonance that gives increased stability compared to other geometric or connective arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability.
In chemistry isomerization or isomerisation is the process in which a molecule, ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the isomerization occurs intramolecularly it may be called a rearrangement reaction.
Azulene is an organic compound and an isomer of naphthalene. Whereas naphthalene is colourless, azulene is dark blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature the azulene skeleton are found in nature as constituents of pigments in mushrooms, guaiac wood oil, and some marine invertebrates.
Cyclodecapentaene or [10]annulene is an annulene with molecular formula C10H10. This organic compound is a conjugated 10 pi electron cyclic system and according to Huckel's rule it should display aromaticity. It is not aromatic, however, because various types of ring strain destabilize an all-planar geometry. The all-cis isomer (1), a fully convex decagon, would have bond angles of 144°, which creates large amounts of angle strain relative to the ideal 120° for sp2 atomic hybridization. Instead, the all-cis isomer can adopt a planar boat-like conformation (2) to relieve the angle strain. This is still unstable because of the relative higher strain in boat shaped compared to the next planar trans, cis, trans, cis, cis isomer (3). Yet even this isomer is also unstable, suffering from steric repulsion between the two internal hydrogen atoms. The nonplanar trans, cis, cis, cis, cis isomer (4) is the most stable of all the possible isomers.

An iminium cation in organic chemistry is a functional group with the general structure [R1R2C=NR3R4]+. They are common in synthetic chemistry and biology.

Guaiazulene, also azulon or 1,4-dimethyl-7-isopropylazulene, is a dark blue crystalline hydrocarbon. A derivative of azulene, guaiazulene is a bicyclic sesquiterpene that is a constituent of some essential oils, mainly oil of guaiac and chamomile oil, which also serve as its commercial sources. Various soft corals also contain guaiazulene as a principal pigment. Its low melting point makes guaiazulene difficult to handle, in contrast to the crystalline nature of the parent azulene. The electronic structure of guaiazulene and azulene are very similar.

Vetivazulene is an azulene derivate obtained from vetiver oil. It is a bicyclic sesquiterpene and an isomer of guaiazulene.
Ring expansion and ring contraction reactions in the course of organic synthesis refer to a set of reactions which can lead to the expansion or contraction of an existing ring. This often makes it possible to access structures that would be difficult if not impossible to synthesise with single cyclization reactions. Ring expansions are valuable because they allow access to larger systems that are difficult to synthesize through a single cyclization due to the slow rate of formation. Ring contractions are useful for making smaller, more strained rings from larger rings. Expansions are classified by the mechanism of expansion and the atom(s) added; contractions are characterized simply by the reactive intermediate which performs the contraction.
Cycloheptatriene (CHT) is an organic compound with the formula C7H8. It is a closed ring of seven carbon atoms joined by three double bonds (as the name implies) and four single bonds. This colourless liquid has been of recurring theoretical interest in organic chemistry. It is a ligand in organometallic chemistry and a building block in organic synthesis. Cycloheptatriene is not aromatic, as reflected by the nonplanarity of the methylene bridge (-CH2-) with respect to the other atoms; however the related tropylium cation is.

Fulvalene (bicyclopentadienylidene) is the member of the fulvalene family with the molecular formula C10H8. It is of theoretical interest as one of the simplest non-benzenoid conjugated hydrocarbons. Fulvalene is an unstable isomer of the more common benzenoid aromatic compounds naphthalene and azulene. Fulvalene consists of two 5-membered rings, each with two double bonds, joined by yet a fifth double bond. It has D2h symmetry.
The molecular formula C10H8 (molar mass: 128.17 g/mol) may refer to:
Walter Julius Reppe was a German chemist. He is notable for his contributions to the chemistry of acetylene.
In organic chemistry, two molecules are valence isomers when they are constitutional isomers that can interconvert through pericyclic reactions.
In organic chemistry, benzenoids are a class of chemical compounds with at least one benzene ring. These compounds have increased stability from resonance in the benzene rings. Most aromatic hydrocarbons are benzenoid. Notable counterexamples are cyclooctadecanonaene, azulene and trans-bicalicene.

Chamazulene is an aromatic chemical compound with the molecular formula C14H16 found in a variety of plants including in chamomile (Matricaria chamomilla), wormwood (Artemisia absinthium), and yarrow (Achillea millefolium). It is a blue-violet derivative of azulene which is biosynthesized from the sesquiterpene matricin.

Werner Tochtermann is a German chemist and emeritus professor. From 1976 till his retirement in 1999 he was full professor of Organic Chemistry at the University of Kiel. His main areas of research were the chemistry of medium and large rings, the synthesis of cyclophanes, and the heteroquadricyclane→heteropin rearrangement, e.g. for the synthesis of oxepins from furans.

HU-243 (AM-4056) is a synthetic cannabinoid drug that is a single enantiomer of the hydrogenated derivative of the commonly used reference agonist HU-210. It is a methylene homologue of canbisol. It is a potent agonist at both the CB1 and CB2 receptors, with a binding affinity of 0.041 nM at the CB1 receptor, making it marginally more potent than HU-210, which had an affinity of 0.061 nM in the same assay.
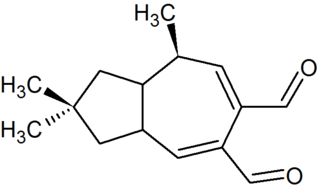
Velleral (2,2,8-trimethyl-3,3a,8,8a-tetrahydro-1H-azulene-5,6-dicarbaldehyde) is a sesquiterpene dialdehyde found in certain mushrooms, like Lactarius torminosus and Lactarius vellereus, after which it was named. The compound is thought to be part of a chemical defense system that protects the mushrooms against predation. First isolated in 1969, and characterized structurally in 1973, velleral has antimicrobial activity. Several syntheses have been devised.
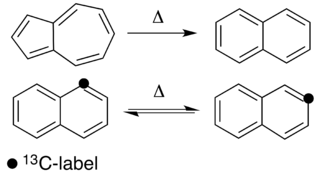
Thermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (isomerization), and one in which the atoms are scrambled but no net change in the aromatic ring occurs (automerization). The general reaction schemes of the two types are illustrated in Figure 1.