Related Research Articles
Isomerases are a general class of enzymes that convert a molecule from one isomer to another. Isomerases facilitate intramolecular rearrangements in which bonds are broken and formed. The general form of such a reaction is as follows:

Galactokinase is an enzyme (phosphotransferase) that facilitates the phosphorylation of α-D-galactose to galactose 1-phosphate at the expense of one molecule of ATP. Galactokinase catalyzes the second step of the Leloir pathway, a metabolic pathway found in most organisms for the catabolism of β-D-galactose to glucose 1-phosphate. First isolated from mammalian liver, galactokinase has been studied extensively in yeast, archaea, plants, and humans.

Methylmalonyl CoA epimerase is an enzyme involved in fatty acid catabolism that is encoded in human by the "MCEE" gene located on chromosome 2. It is routinely and incorrectly labeled as "methylmalonyl-CoA racemase". It is not a racemase because the CoA moiety has 5 other stereocenters.
Galactose-1-phosphate uridylyltransferase deficiency(classic galactosemia), is the most common type of galactosemia, an inborn error of galactose metabolism, caused by a deficiency of the enzyme galactose-1-phosphate uridylyltransferase. It is an autosomal recessive metabolic disorder that can cause liver disease and death if untreated. Treatment of galactosemia is most successful if initiated early and includes dietary restriction of lactose intake. Because early intervention is key, galactosemia is included in newborn screening programs in many areas. On initial screening, which often involves measuring the concentration of galactose in blood, classic galactosemia may be indistinguishable from other inborn errors of galactose metabolism, including galactokinase deficiency and galactose epimerase deficiency. Further analysis of metabolites and enzyme activities are needed to identify the specific metabolic error.
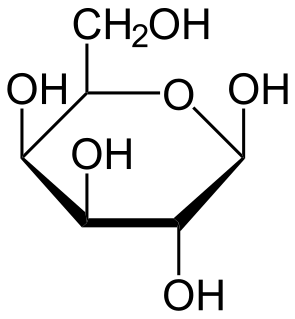
In enzymology, an aldose 1-epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, an amino-acid racemase is an enzyme that catalyzes the chemical reaction
In enzymology, a CDP-paratose 2-epimerase is an enzyme that catalyzes the chemical reaction

In enzymology, a dTDP-4-dehydrorhamnose 3,5-epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, a GDP-mannose 3,5-epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, a glucose-6-phosphate 1-epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, an UDP-arabinose 4-epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, an UDP-glucosamine 4-epimerase is an enzyme that catalyzes the chemical reaction

The enzyme UDP-glucose 4-epimerase, also known as UDP-galactose 4-epimerase or GALE, is a homodimeric epimerase found in bacterial, fungal, plant, and mammalian cells. This enzyme performs the final step in the Leloir pathway of galactose metabolism, catalyzing the reversible conversion of UDP-galactose to UDP-glucose. GALE tightly binds nicotinamide adenine dinucleotide (NAD+), a co-factor required for catalytic activity.
In enzymology, an UDP-glucuronate 4-epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, an UDP-glucuronate 5'-epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, an UDP-N-acetylglucosamine 2-epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, an UDP-N-acetylglucosamine 4-epimerase is an enzyme that catalyzes the chemical reaction
The gal operon is a prokaryotic operon, which encodes enzymes necessary for galactose metabolism. Repression of gene expression for this operon works via binding of repressor molecules to two operators. These repressors dimerize, creating a loop in the DNA. The loop as well as hindrance from the external operator prevent RNA polymerase from binding to the promoter, and thus prevent transcription. Additionally, since the metabolism of galactose in the cell is involved in both anabolic and catabolic pathways, a novel regulatory system using two promoters for differential repression has been identified and characterized within the context of the gal operon.

The Leloir pathway is a metabolic pathway for the catabolism of D-galactose. It is named after Luis Federico Leloir, who first described it.
References
- ↑ Tanner, ME. (2002). "Understanding nature's strategies for enzyme-catalyzed racemization and epimerization". Acc. Chem. Res. 35 (4): 237–246. doi:10.1021/ar000056y. PMID 11955052.
- ↑ "Isomerase | enzyme | Britannica".