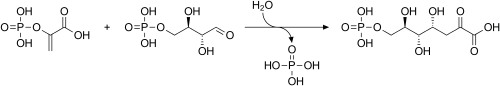| |
 | |
| Names | |
|---|---|
| IUPAC names [(2R,3R)-2,3-Dihydroxy-4-oxobutyl] phosphate d-Erythrose 4-(dihydrogen phosphate) | |
| Other names E4P | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| MeSH | erythrose+4-phosphate |
PubChem CID | |
| UNII | |
| |
| |
| Properties | |
| C4H9O7P | |
| Molar mass | 200.084 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Erythrose 4-phosphate is a phosphate of the simple sugar erythrose. It is an intermediate in the pentose phosphate pathway and the Calvin cycle. [1]
The enzyme transaldolase catalyzes the formation of erythrose 4-phosphate and fructose 6-phosphate from sedoheptulose 7-phosphate and glyceraldehyde 3-phosphate. [2] This reaction is a part of the non-oxidative phase of the pentose phosphate pathway.
In the Calvin cycle, the enzyme fructose-bisphosphate aldolase catalyzes the formation of sedoheptulose 1,7-bisphosphate from erythrose 4-phosphate and dihydroxyacetone phosphate. [3]
In addition, it serves as a precursor in the biosynthesis of the aromatic amino acids tyrosine, phenylalanine, and tryptophan. It is used in the first step of the shikimate pathway. At this stage, phosphoenolpyruvate and erythrose-4-phosphate react to form 3-deoxy-D-arabinoheptulosonate-7-phosphate (DAHP), in a reaction catalyzed by the enzyme DAHP synthase.
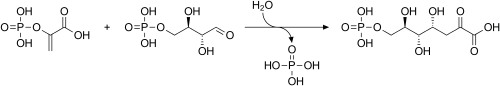
Biosynthesis of DAHP from phosphoenolpyruvate and erythrose-4-phosphate
It also used in 3-hydroxy-1-aminoacetone phosphate biosynthesis, which is a precursor of vitamin B6 in DXP-dependent pathway. Erythrose-4-phosphate dehydrogenase is used to produce 4-phospho-D-erythronic acid: [4] [5]