 | |
| Names | |
|---|---|
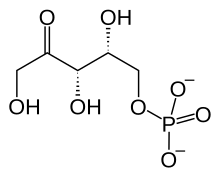
| IUPAC name 5-O-Phosphonato-D-xylulose | |
| Systematic IUPAC name d-threo-Pent-2-ulose 5-phosphate [1] | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| MeSH | xylulose-5-phosphate |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C5H11O8P | |
| Molar mass | 230.109 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
D-Xylulose 5-phosphate (D-xylulose-5-P) is an intermediate in the pentose phosphate pathway. It is a ketose sugar formed from ribulose-5-phosphate by ribulose-5-phosphate epimerase. In the non-oxidative branch of the pentose phosphate pathway, xylulose-5-phosphate acts as a donor of two-carbon ketone groups in transketolase reactions. [2]
Xylulose-5-phosphate also plays a crucial role in the regulation of glycolysis through its interaction with the bifunctional enzyme PFK2/FBPase2. Specifically, it activates protein phosphatase, which then dephosphorylates PFK2/FBPase2. This inactivates the FBPase2 activity of the bifunctional enzyme and activates its PFK2 activity. [3] As a result, the production of fructose 2,6-bisphosphate increases, ultimately leading to an upregulation of glycolysis. [4]
Although previously thought of mainly as an intermediary in the pentose phosphate pathway, recent research reported that the sugar also has a role in gene expression, mainly by promoting the ChREBP transcription factor in the well-fed state. [5] [6] However, more recent study showed that D-glucose-6-phosphate, rather than D-xylulose-5-phosphate, is essential for the activation of ChREBP in response to glucose. [7]