
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Carbon is the main component of biological compounds as well as a major component of many minerals such as limestone. Along with the nitrogen cycle and the water cycle, the carbon cycle comprises a sequence of events that are key to make Earth capable of sustaining life. It describes the movement of carbon as it is recycled and reused throughout the biosphere, as well as long-term processes of carbon sequestration to and release from carbon sinks. Carbon sinks in the land and the ocean each currently take up about one-quarter of anthropogenic carbon emissions each year.

An estuary is a partially enclosed coastal body of brackish water with one or more rivers or streams flowing into it, and with a free connection to the open sea. Estuaries form a transition zone between river environments and maritime environments and are an example of an ecotone. Estuaries are subject both to marine influences such as tides, waves, and the influx of saline water, and to fluvial influences such as flows of freshwater and sediment. The mixing of seawater and freshwater provides high levels of nutrients both in the water column and in sediment, making estuaries among the most productive natural habitats in the world.

The biological pump (or ocean carbon biological pump or marine biological carbon pump) is the ocean's biologically driven sequestration of carbon from the atmosphere and land runoff to the ocean interior and seafloor sediments. In other words, it is a biologically mediated processes which result in the sequestering of carbon in the deep ocean away from the atmosphere and the land. The biological pump is the biological component of the "marine carbon pump" which contains both a physical and biological component. It is the part of the broader oceanic carbon cycle responsible for the cycling of organic matter formed mainly by phytoplankton during photosynthesis (soft-tissue pump), as well as the cycling of calcium carbonate (CaCO3) formed into shells by certain organisms such as plankton and mollusks (carbonate pump).
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particle-free space. Due to its extremely high charge density of approximately 2×1010 times that of a sodium ion, the bare hydrogen ion cannot exist freely in solution as it readily hydrates, i.e., bonds quickly. The hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes. Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions.

In oceanic biogeochemistry, the solubility pump is a physico-chemical process that transports carbon as dissolved inorganic carbon (DIC) from the ocean's surface to its interior.

Dissolved inorganic carbon (DIC) is the sum of the aqueous species of inorganic carbon in a solution. Carbon compounds can be distinguished as either organic or inorganic, and as dissolved or particulate, depending on their composition. Organic carbon forms the backbone of key component of organic compounds such as – proteins, lipids, carbohydrates, and nucleic acids.
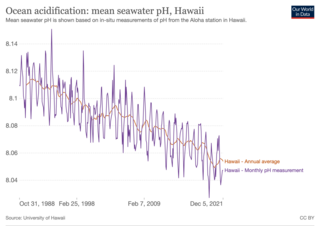
Ocean acidification is the reduction in the pH value of the Earth’s ocean. Between 1751 and 2021, the average pH value of the ocean surface has decreased from approximately 8.25 to 8.14. The root cause of ocean acidification is carbon dioxide emissions from human activities which have led to atmospheric carbon dioxide (CO2) levels of more than 410 ppm (in 2020). The oceans absorb CO2 from the atmosphere. This leads to the formation of carbonic acid (H2CO3) which dissociates into a bicarbonate ion (HCO−3) and a hydrogen ion (H+). The free hydrogen ions (H+) decrease the pH of the ocean, therefore increasing acidity (this does not mean that seawater is acidic yet; it is still alkaline, with a pH higher than 8). A decrease in pH corresponds to a decrease in the concentration of carbonate ions, which are the main building block for calcium carbonate (CaCO3) shells and skeletons. Marine calcifying organisms, like mollusks, oysters and corals, are particularly affected by this as they rely on calcium carbonate to build shells and skeletons.

Ocean deoxygenation is the reduction of the oxygen content of the global oceans and coastal zones due to human activities as a consequence of anthropogenic emissions of carbon dioxide and eutrophication-driven excess production. It is manifest in the increasing number of coastal and estuarine hypoxic areas, or dead zones, and the expansion of oxygen minimum zones (OMZs) in the world's oceans. The decrease in oxygen content of the oceans has been fairly rapid and poses a threat to all aerobic marine life, as well as to people who depend on marine life for nutrition or livelihood.
Marine chemistry, also known as ocean chemistry or chemical oceanography, is influenced by plate tectonics and seafloor spreading, turbidity currents, sediments, pH levels, atmospheric constituents, metamorphic activity, and ecology. The field of chemical oceanography studies the chemistry of marine environments including the influences of different variables. Marine life has adapted to the chemistries unique to earth's oceans, and marine ecosystems are sensitive to changes in ocean chemistry.

Hypoxia refers to low oxygen conditions. Normally, 20.9% of the gas in the atmosphere is oxygen. The partial pressure of oxygen in the atmosphere is 20.9% of the total barometric pressure. In water, oxygen levels are much lower, approximately 7 ppm or 0.0007% in good quality water, and fluctuate locally depending on the presence of photosynthetic organisms and relative distance to the surface.

The oceanic carbon cycle is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor. The carbon cycle is a result of many interacting forces across multiple time and space scales that circulates carbon around the planet, ensuring that carbon is available globally. The Oceanic carbon cycle is a central process to the global carbon cycle and contains both inorganic carbon and organic carbon. Part of the marine carbon cycle transforms carbon between non-living and living matter.

Shell growth in estuaries is an aspect of marine biology that has attracted a number of scientific research studies. Many groups of marine organisms produce calcified exoskeletons, commonly known as shells, hard calcium carbonate structures which the organisms rely on for various specialized structural and defensive purposes. The rate at which these shells form is greatly influenced by physical and chemical characteristics of the water in which these organisms live. Estuaries are dynamic habitats which expose their inhabitants to a wide array of rapidly changing physical conditions, exaggerating the differences in physical and chemical properties of the water.
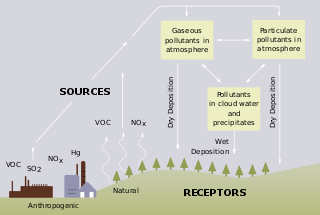
Freshwater acidification occurs when acidic inputs enter a body of fresh water through the weathering of rocks, invasion of acidifying gas, or by the reduction of acid anions, like sulfate and nitrate within the lake. Freshwater acidification is primarily caused by sulfur oxides (SOx) and nitrogen oxides (NOx) entering the water from atmospheric depositions and soil leaching. Carbonic acid and dissolved carbon dioxide can also enter freshwaters in a similar manner associated with runoff through carbon dioxide-rich soils. Runoff that contains these compounds may be accompanied by acidifying hydrogen ions and inorganic aluminum, which can be toxic to marine organisms. Acid rain is also a contributor to freshwater acidification. It is created when SOx and NOx react with water, oxygen, and other oxidants within the clouds.
Ocean acidification threatens the Great Barrier Reef by reducing the viability and strength of coral reefs. The Great Barrier Reef, considered one of the seven natural wonders of the world and a biodiversity hotspot, is located in Australia. Similar to other coral reefs, it is experiencing degradation due to ocean acidification. Ocean acidification results from a rise in atmospheric carbon dioxide, which is taken up by the ocean. This process can increase sea surface temperature, decrease aragonite, and lower the pH of the ocean. The more humanity consumes fossil fuels, the more the ocean absorbs released CO₂, furthering ocean acidification.
The Southern Ocean Carbon and Climate Observations and Modeling (SOCCOM) project is a large scale National Science Foundation funded research project based at Princeton University that started in September 2014. The project aims to increase the understanding of the Southern Ocean and the role it plays in factors such as climate, as well as educate new scientists with oceanic observation.
Nutrient cycling in the Columbia River Basin involves the transport of nutrients through the system, as well as transformations from among dissolved, solid, and gaseous phases, depending on the element. The elements that constitute important nutrient cycles include macronutrients such as nitrogen, silicate, phosphorus, and micronutrients, which are found in trace amounts, such as iron. Their cycling within a system is controlled by many biological, chemical, and physical processes.

The Arctic ocean covers an area of 14,056,000 squared kilometers, and supports a diverse and important socioeconomic food web of organisms, despite its average water temperature being 32 degrees Fahrenheit. Over the last three decades, the Arctic Ocean has experienced drastic changes due to climate change. One of the changes is in the acidity levels of the ocean, which have been consistently increasing at twice the rate of the Pacific and Atlantic oceans. Arctic Ocean acidification is a result of feedback from climate system mechanisms, and is having negative impacts on Arctic Ocean ecosystems and the organisms that live within them.

Human activities affect marine life and marine habitats through overfishing, habitat loss, the introduction of invasive species, ocean pollution, ocean acidification and ocean warming. These impact marine ecosystems and food webs and may result in consequences as yet unrecognised for the biodiversity and continuation of marine life forms.

Jean-Pierre Gattuso is a French ocean scientist conducting research globally, from the pole to the tropics and from nearshore to the open ocean. His research addresses the biology of reef-building corals, the biogeochemistry of coastal ecosystems, and the response of marine plants, animals and ecosystems to global environmental change. He is also interested in transdisciplinary research, collaborating with social scientists to address ocean-based solutions to minimize climate change and its impacts. He is currently a CNRS Research Professor at Sorbonne University.

Particulate inorganic carbon (PIC) can be contrasted with dissolved inorganic carbon (DIC), the other form of inorganic carbon found in the ocean. These distinctions are important in chemical oceanography. Particulate inorganic carbon is sometimes called suspended inorganic carbon. In operational terms, it is defined as the inorganic carbon in particulate form that is too large to pass through the filter used to separate dissolved inorganic carbon.















