MOLPRO is a software package used for accurate ab initio quantum chemistry calculations. It is developed by Peter Knowles at Cardiff University and Hans-Joachim Werner at Universität Stuttgart in collaboration with other authors.
In computational chemistry, post–Hartree–Fock (post-HF) methods are the set of methods developed to improve on the Hartree–Fock (HF), or self-consistent field (SCF) method. They add electron correlation which is a more accurate way of including the repulsions between electrons than in the Hartree–Fock method where repulsions are only averaged.
In chemistry, a carbonium ion is any cation that has a pentacoordinated carbon atom. The name carbonium may also be used for the simplest member of the class, properly called methanium, where the carbon atom is covalently bonded to five hydrogen atoms.

Spartan is a molecular modelling and computational chemistry application from Wavefunction. It contains code for molecular mechanics, semi-empirical methods, ab initio models, density functional models, post-Hartree–Fock models, and thermochemical recipes including G3(MP2) and T1. Quantum chemistry calculations in Spartan are powered by Q-Chem.
John "Jack" Gamble Kirkwood was a noted chemist and physicist, holding faculty positions at Cornell University, the University of Chicago, California Institute of Technology, and Yale University.

Carbon trioxide (CO3) is an unstable oxide of carbon (an oxocarbon). The possible isomers of carbon trioxide include ones with molecular symmetry point groups Cs, D3h, and C2v. The C2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. Carbon trioxide should not be confused with the stable carbonate ion (CO2−
3).
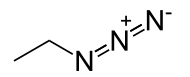
A harpoon reaction is a type of chemical reaction, first proposed by Michael Polanyi in 1920, whose mechanism involves two neutral reactants undergoing an electron transfer over a relatively long distance to form ions that then attract each other closer together. For example, a metal atom and a halogen might react to form a cation and anion, respectively, leading to a combined metal halide.

In computational chemistry, a water model is used to simulate and thermodynamically calculate water clusters, liquid water, and aqueous solutions with explicit solvent. The models are determined from quantum mechanics, molecular mechanics, experimental results, and these combinations. To imitate a specific nature of molecules, many types of models have been developed. In general, these can be classified by the following three points; (i) the number of interaction points called site, (ii) whether the model is rigid or flexible, (iii) whether the model includes polarization effects.
This page deals with the electron affinity as a property of isolated atoms or molecules. Solid state electron affinities are not listed here.
Path integral Monte Carlo (PIMC) is a quantum Monte Carlo method used to solve quantum statistical mechanics problems numerically within the path integral formulation. The application of Monte Carlo methods to path integral simulations of condensed matter systems was first pursued in a key paper by John A. Barker.

Ice VII is a cubic crystalline form of ice. It can be formed from liquid water above 3 GPa (30,000 atmospheres) by lowering its temperature to room temperature, or by decompressing heavy water (D2O) ice VI below 95 K. (Different types of ice, from ice II to ice XVIII, have been created in the laboratory at different temperatures and pressures. Ordinary water ice is known as ice Ih in the Bridgman nomenclature.) Ice VII is metastable over a wide range of temperatures and pressures and transforms into low-density amorphous ice (LDA) above 120 K (−153 °C). Ice VII has a triple point with liquid water and ice VI at 355 K and 2.216 GPa, with the melt line extending to at least 715 K (442 °C) and 10 GPa. Ice VII can be formed within nanoseconds by rapid compression via shock-waves. It can also be created by increasing the pressure on ice VI at ambient temperature. At around 5 GPa, Ice VII becomes the tetragonal Ice VIIt.

Malcolm Dole was an American chemist known for the Dole Effect in which he proved that the atomic weight of oxygen in air is greater than that of oxygen in water and for his work on electrospray ionization, polymer chemistry, and electrochemistry.

HCNH+, also known as protonated hydrogen cyanide, is a molecular ion of astrophysical interest. It also exists in the condensed state when formed by superacids.
In computer software, FreeON is an experimental, open source (GPL) suite of programs for linear scaling quantum chemistry, formerly known as MondoSCF. It is highly modular, and has been written from scratch for N-scaling SCF theory in Fortran95 and C. Platform independent IO is supported with HDF5. FreeON should compile with most modern Linux distributions. FreeON performs Hartree–Fock, pure density functional, and hybrid HF/DFT calculations in a Cartesian-Gaussian LCAO basis. All algorithms are O(N) or O(N lg N) for non-metallic systems. Periodic boundary conditions in 1, 2 and 3 dimensions have been implemented through the Lorentz field, and an internal coordinate geometry optimizer allows full (atom+cell) relaxation using analytic derivatives. Effective core potentials for energies and forces have been implemented, but Effective Core Potential (ECP) lattice forces do not work yet. Advanced features include O(N) static and dynamic response, as well as time reversible Born Oppenheimer Molecular Dynamics (MD).
Helium is the smallest and the lightest noble gas and one of the most unreactive elements, so it was commonly considered that helium compounds cannot exist at all, or at least under normal conditions. Helium's first ionization energy of 24.57 eV is the highest of any element. Helium has a complete shell of electrons, and in this form the atom does not readily accept any extra electrons nor join with anything to make covalent compounds. The electron affinity is 0.080 eV, which is very close to zero. The helium atom is small with the radius of the outer electron shell at 0.29 Å. Helium is a very hard atom with a Pearson hardness of 12.3 eV. It has the lowest polarizability of any kind of atom, however, very weak van der Waals forces exist between helium and other atoms. This force may exceed repulsive forces, so at extremely low temperatures helium may form van der Waals molecules. Helium has the lowest boiling point of any known substance.
Neon compounds are chemical compounds containing the element neon (Ne) with other molecules or elements from the periodic table. Compounds of the noble gas neon were believed not to exist, but there are now known to be molecular ions containing neon, as well as temporary excited neon-containing molecules called excimers. Several neutral neon molecules have also been predicted to be stable, but are yet to be discovered in nature. Neon has been shown to crystallize with other substances and form clathrates or Van der Waals solids.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.
Thorium monoxide, is the binary oxide of thorium having chemical formula ThO. The covalent bond in this diatomic molecule is highly polar. The effective electric field between the two atoms has been calculated to be about 80 gigavolts per centimeter, one of the largest known internal effective electric fields.
Erin Johnson is a Canadian computational chemist. She holds the Herzberg–Becke Chair at Dalhousie University. She works on density functional theory and intermolecular interactions.

John Lafayette Magee was an American chemist known for his work on kinetic models of radiation chemistry, especially the Samuel-Magee model for describing radiolysis in solution.