
Griseofulvin is an antifungal medication used to treat a number of types of dermatophytoses (ringworm). This includes fungal infections of the nails and scalp, as well as the skin when antifungal creams have not worked. It is taken by mouth.
The Fischer indole synthesis is a chemical reaction that produces the aromatic heterocycle indole from a (substituted) phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Emil Fischer. Today antimigraine drugs of the triptan class are often synthesized by this method.

The Baker–Venkataraman rearrangement is the chemical reaction of 2-acetoxyacetophenones with base to form 1,3-diketones.

Lisofylline (LSF) is a synthetic small molecule with novel anti-inflammatory properties. LSF can effectively prevent type 1 diabetes in preclinical models and improves the function and viability of isolated or transplanted pancreatic islets. It is a metabolite of pentoxifylline.

Tropolone is an organic compound with the chemical formula C7H5(OH)O. It is a pale yellow solid that is soluble in organic solvents. The compound has been of interest to research chemists because of its unusual electronic structure and its role as a ligand precursor. Although not usually prepared from tropone, it can be viewed as its derivative with a hydroxyl group in the 2-position.

Gliotoxin is a sulfur-containing mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines produced by several species of fungi, especially those of marine origin. It is the most prominent member of the epipolythiopiperazines, a large class of natural products featuring a diketopiperazine with di- or polysulfide linkage. These highly bioactive compounds have been the subject of numerous studies aimed at new therapeutics. Gliotoxin was originally isolated from Gliocladium fimbriatum, and was named accordingly. It is an epipolythiodioxopiperazine metabolite that is one of the most abundantly produced metabolites in human invasive Aspergillosis (IA).

Cycloguanil is a dihydrofolate reductase inhibitor, and is a metabolite of the antimalarial drug proguanil; its formation in vivo has been thought to be primarily responsible for the antimalarial activity of proguanil. However, more recent work has indicated that, while proguanil is synergistic with the drug atovaquone, cycloguanil is in fact antagonistic to the effects of atovaquone, suggesting that, unlike cycloguanil, proguanil may have an alternative mechanism of antimalarial action besides dihydrofolate reductase inhibition.

Larry E. Overman is Distinguished Professor of Chemistry at the University of California, Irvine. He was born in Chicago in 1943. Overman obtained a B.A. degree from Earlham College in 1965, and he completed his Ph.D. in chemistry from the University of Wisconsin–Madison in 1969, under Howard Whitlock Jr. Professor Overman is a member of the United States National Academy of Sciences and the American Academy of Arts and Sciences. He was the recipient of the Arthur C. Cope Award in 2003, and he was awarded the Tetrahedron Prize for Creativity in Organic Chemistry for 2008.

Bergapten (5-methoxypsoralen) is a naturally-occurring organic chemical compound produced by numerous plant species, especially from the carrot family Apiaceae and the citrus family Rutaceae. For example, bergapten has been extracted from 24 species of the genus Heracleum in the family Apiaceae. In the family Rutaceae, various Citrus species contain significant amounts of bergapten, especially the bergamot orange, the micrantha, and certain varieties of lime and bitter orange.
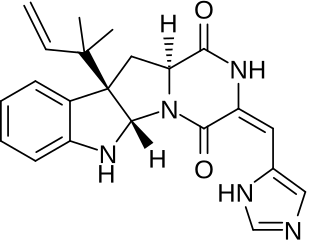
Roquefortine C is a mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines produced by various fungi, particularly species from the genus Penicillium. It was first isolated from a strain of Penicillium roqueforti, a species commercially used as a source of proteolytic and lipolytic enzymes during maturation of the blue-veined cheeses, Roquefort, Danish Blue, Stilton and Gorgonzola.

Brevianamides are indole alkaloids that belong to a class of naturally occurring 2,5-diketopiperazines produced as secondary metabolites of fungi in the genus Penicillium and Aspergillus. Structurally similar to paraherquamides, they are a small class compounds that contain a bicyclo[2.2.2]diazoctane ring system. One of the major secondary metabolites in Penicillium spores, they are responsible for inflammatory response in lung cells.

Altechromone A is a chromone derivative. To date, it has been isolated from plant families such as Polygonaceae, Lamiaceae, Fabaceae, and Hypericaceae.

The Kostanecki acylation is a method used in organic synthesis to form chromones or coumarins by acylation of O-hydroxyaryl ketones with aliphatic acid anhydrides, followed by cyclization. If benzoic anhydride is used, a particular type of chromone called a flavone is obtained.

Visnagin is an organic chemical compound with the molecular formula C13H10O4 It is a furanochromone, a compound derivative of chromone (1,4-benzopyrone) and furan.

Stephacidin A and B are antitumor alkaloids isolated from the fungus Aspergillus ochraceus that belong to a class of naturally occurring 2,5-diketopiperazines. This unusual family of fungal metabolites are complex bridged 2,5-diketopiperazine alkaloids that possess a unique bicyclo[2.2.2]diazaoctane core ring system and are constituted mainly from tryptophan, proline, and substituted proline derivatives where the olefinic unit of the isoprene moiety has been formally oxidatively cyclized across the α-carbon atoms of a 2,5-diketopiperazine ring. The molecular architecture of stephacidin B, formally a dimer of avrainvillamide, reveals a complex dimeric prenylated N-hydroxyindole alkaloid that contains 15 rings and 9 stereogenic centers and is one of the most complex indole alkaloids isolated from fungi. Stephacidin B rapidly converts into the electrophilic monomer avrainvillamide in cell culture, and there is evidence that the monomer avrainvillamide interacts with intracellular thiol-containing proteins, most likely by covalent modification.
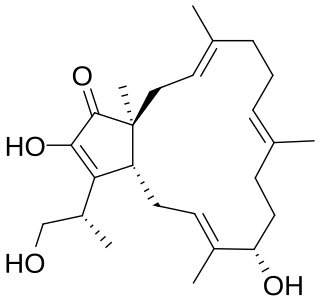
Terpestacin is a fungal metabolite first isolated from Arthrinium.

Mitragynine pseudoindoxyl is a rearrangement product of 7-hydroxymitragynine, an active metabolite of mitragynine.

Ethylestradiol, or 17α-ethylestradiol, also known as 17α-ethylestra-1,3,5(10)-triene-3,17β-diol, is a synthetic estrogen which was never marketed. It occurs as an active metabolite of the anabolic steroids norethandrolone and ethylestrenol formed via aromatase and is believed to be responsible for the estrogenic effects of norethandrolone and ethylestrenol. The 3-methyl ether of ethylestradiol has been used as an intermediate in the synthesis of certain 19-nortestosterone anabolic steroids.

Total mycosynthesis is the combination of the use of a filamentous fungal host organism with a genetic expression system that allows the assembly and controlled expression of one or more biosynthetic genes. Total mycosynthesis involves the reconstruction and/or engineering of biosynthetic pathways for the production of secondary metabolites. It is competitive with chemical total synthesis. It can be used both for the production of known natural products, and for the engineering of pathways to produce new compounds or pathway intermediates.
Lichen products, also known as lichen substances, are organic compounds produced by a lichen. Specifically, they are secondary metabolites. Lichen products are represented in several different chemical classes, including terpenoids, orcinol derivatives, chromones, xanthones, depsides, and depsidones. Over 800 lichen products of known chemical structure have been reported in the scientific literature, and most of these compounds are exclusively found in lichens. Examples of lichen products include usnic acid, atranorin, lichexanthone, salazinic acid, and isolichenan, an α-glucan. Many lichen products have biological activity, and research into these effects is ongoing.


















