Apiaceae or Umbelliferae is a family of mostly aromatic flowering plants named after the type genus Apium and commonly known as the celery, carrot or parsley family, or simply as umbellifers. It is the 16th-largest family of flowering plants, with more than 3,800 species in about 446 genera, including such well-known and economically important plants as ajwain, angelica, anise, asafoetida, caraway, carrot, celery, chervil, coriander, cumin, dill, fennel, lovage, cow parsley, parsley, parsnip and sea holly, as well as silphium, a plant whose identity is unclear and which may be extinct.

The parsnip is a root vegetable closely related to carrot and parsley, all belonging to the flowering plant family Apiaceae. It is a biennial plant usually grown as an annual. Its long taproot has cream-colored skin and flesh, and, left in the ground to mature, becomes sweeter in flavor after winter frosts. In its first growing season, the plant has a rosette of pinnate, mid-green leaves. If unharvested, it produces a flowering stem topped by an umbel of small yellow flowers in its second growing season, later producing pale brown, flat, winged seeds. By this time, the stem has become woody, and the tap root inedible. Precautions should be taken when handling the stems and foliage, as parsnip sap can cause a skin rash or even blindness if exposed to sunlight after handling.

Falcarinol is a natural pesticide and fatty alcohol found in carrots, red ginseng and ivy. In carrots, it occurs in a concentration of approximately 2 mg/kg. As a toxin, it protects roots from fungal diseases, such as liquorice rot that causes black spots on the roots during storage. The compound requires the freezing condition to maintain well because it is sensitive to light and heat.

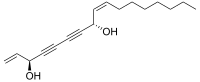
Cicutoxin is a naturally-occurring poisonous chemical compound produced by several plants from the family Apiaceae including water hemlock (Cicuta species) and water dropwort (Oenanthe crocata). The compound contains polyene, polyyne, and alcohol functional groups and is a structural isomer of oenanthotoxin, also found in water dropwort. Both of these belong to the C17-polyacetylenes chemical class.

Gingerol ([6]-gingerol) is a phenolic phytochemical compound found in fresh ginger that activates heat receptors on the tongue. It is normally found as a pungent yellow oil in the ginger rhizome, but can also form a low-melting crystalline solid. This chemical compound is found in all members of the Zingiberaceae family and is high in concentrations in the grains of paradise as well as an African Ginger species.

A polyyne is any organic compound with alternating single and triple bonds; that is, a series of consecutive alkynes, (−C≡C−)n with n greater than 1. These compounds are also called polyacetylenes, especially in the natural products and chemical ecology literature, even though this nomenclature more properly refers to acetylene polymers composed of alternating single and double bonds (−CR=CR′−)n with n greater than 1. They are also sometimes referred to as oligoynes, or carbinoids after "carbyne" (−C≡C−)∞, the hypothetical allotrope of carbon that would be the ultimate member of the series. The synthesis of this substance has been claimed several times since the 1960s, but those reports have been disputed. Indeed, the substances identified as short chains of "carbyne" in many early organic synthesis attempts would be called polyynes today.

Diallyl disulfide is an organosulfur compound derived from garlic and a few other genus Allium plants. Along with diallyl trisulfide and diallyl tetrasulfide, it is one of the principal components of the distilled oil of garlic. It is a yellowish liquid which is insoluble in water and has a strong garlic odor. It is produced during the decomposition of allicin, which is released upon crushing garlic and other plants of the family Alliaceae. Diallyl disulfide has many of the health benefits of garlic, but it is also an allergen causing garlic allergy. Highly diluted, it is used as a flavoring in food. It decomposes in the human body into other compounds such as allyl methyl sulfide.

The carrot is a root vegetable, typically orange in color, though purple, black, red, white, and yellow cultivars exist, all of which are domesticated forms of the wild carrot, Daucus carota, native to Europe and Southwestern Asia. The plant probably originated in Persia and was originally cultivated for its leaves and seeds. The most commonly eaten part of the plant is the taproot, although the stems and leaves are also eaten. The domestic carrot has been selectively bred for its enlarged, more palatable, less woody-textured taproot.

Ursolic acid, is a pentacyclic triterpenoid identified in the epicuticular waxes of apples as early as 1920 and widely found in the peels of fruits, as well as in herbs and spices like rosemary and thyme.

Emodin (6-methyl-1,3,8-trihydroxyanthraquinone) is a chemical compound, of the anthraquinone family, that can be isolated from rhubarb, buckthorn, and Japanese knotweed. Emodin is particularly abundant in the roots of the Chinese rhubarb, knotweed and knotgrass as well as Hawaii ‘au‘auko‘i cassia seeds or coffee weed. It is specifically isolated from Rheum palmatum L. It is also produced by many species of fungi, including members of the genera Aspergillus, Pyrenochaeta, and Pestalotiopsis, inter alia. The common name is derived from Rheum emodi, a taxonomic synonym of Rheum australe, and synonyms include emodol, frangula emodin, rheum emodin, 3-methyl-1,6,8-trihydroxyanthraquinone, Schüttgelb (Schuttgelb), and Persian Berry Lake.
Mycocentrospora acerina is a deuteromycete fungus that is a plant pathogen.

Magnolol is an organic compound that is classified as lignan. It is a bioactive compound found in the bark of the Houpu magnolia or in M. grandiflora. The compound exists at the level of a few percent in the bark of species of magnolia, the extracts of which have been used in traditional Chinese and Japanese medicine. In addition to magnolol, related lignans occur in the extracts including honokiol, which is an isomer of magnolol.
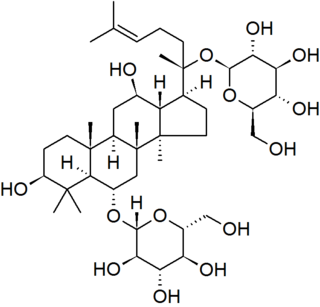
Ginsenosides or panaxosides are a class of natural product steroid glycosides and triterpene saponins. Compounds in this family are found almost exclusively in the plant genus Panax (ginseng), which has a long history of use in traditional medicine that has led to the study of pharmacological effects of ginseng compounds. As a class, ginsenosides exhibit a large variety of subtle and difficult-to-characterize biological effects when studied in isolation.

Berula erecta, known as lesser water-parsnip or cutleaf waterparsnip or narrow-leaved water-parsnip, is a member of the carrot family. Growing to around 1 m (3 ft) tall, it is found in or by water. It is widespread across much of Europe, Asia, Australia, and North America.

Xanthohumol is a natural product found in the female inflorescences of Humulus lupulus, also known as hops. This compound is also found in beer and belongs to a class of compounds that contribute to the bitterness and flavor of hops. Xanthohumol is a prenylated chalconoid, biosynthesized by a type III polyketide synthase (PKS) and subsequent modifying enzymes.

In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.
Hansenia weberbaueriana, synonym Notopterygium incisum, is a species of flowering plant in the family Apiaceae, native from northeastern Tibet to central China. It is known as the notopterygium root. Its root and rhizome parts of the plant have long been used in traditional Chinese medicine, and the plant continues to be thoroughly studied to this day. As a result of its use, it has become endangered due to excessive cultivation for use in commercial products.
A bioactive compound is a compound that has an effect on a living organism, tissue or cell, usually demonstrated by basic research in vitro or in vivo in the laboratory. While dietary nutrients are essential to life, bioactive compounds have not been proved to be essential – as the body can function without them – or because their actions are obscured by nutrients fulfilling the function.
1,7-Bis(4-hydroxyphenyl)-1,4,6-heptatrien-3-one is a natural product, a curcuminoid antioxidant found in turmeric and torch ginger.

Pseudoginsenoside F11 is a chemical natural product found in American ginseng but not in Asian ginseng, although it has similar properties to the Asian ginseng compound ginsenoside Rf. The molecule is a triterpenoid saponin member of the dammarane family and contains a four-ring rigid skeleton. Compounds in the ginsenoside family are found almost exclusively in plants of the genus Panax. A wide variety of difficult-to-characterize in vitro effects have been reported for the compounds in isolation. Pseudoginsenoside F11 and its derivatives are sometimes referred to as having an ocotillol-type skeleton structure.