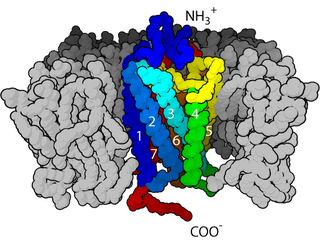
Fasciclin 2 (Fas2 or FasII) [1] is a 95 kilodalton cell membrane glycoprotein in the immunoglobulin (Ig) – related superfamily of cell adhesion molecules (CAMs). [2] It was first identified in the developing grasshopper embryo, seen dynamically expressed on a subset of fasciculating axons in the central nervous system (CNS), functioning as a neuronal recognition molecule in the regulation of selective axon fasciculation. [2] Subsequently, fasII was cloned and has mainly been studied in the fruit fly ( Drosophila melanogaster ). [2] Its extracellular structure consists of two Fibronectin type III domains and five Ig-like C2 domains, having structural homology to the neural cell adhesion molecule (NCAM) found in vertebrates. [2] Alternative splicing of fasII gives rise to its expression in three major isoforms, including a membrane-associated form that is attached to the outer leaflet of the plasma membrane via a glycophosphatidylinositol (GPI anchor) linkage and two integral transmembrane forms. [2] The larger transmembrane form has an amino acid motif contained in its cytoplasmic domain that is rich in proline, glutamic acid, serine and threonine residues (PEST sequence). [2] The fasciclin 1 (Fas1) and fasciclin 3 (Fas3) genes in Drosophila also code for cell adhesion proteins in the nervous system but do not show any structural or functional similarities with NCAM. [2]

The cell membrane is a biological membrane that separates the interior of all cells from the outside environment which protects the cell from its environment consisting of a lipid bilayer with embedded proteins. The cell membrane controls the movement of substances in and out of cells and organelles. In this way, it is selectively permeable to ions and organic molecules. In addition, cell membranes are involved in a variety of cellular processes such as cell adhesion, ion conductivity and cell signalling and serve as the attachment surface for several extracellular structures, including the cell wall, the carbohydrate layer called the glycocalyx, and the intracellular network of protein fibers called the cytoskeleton. In the field of synthetic biology, cell membranes can be artificially reassembled.

Glycoproteins are proteins which contain oligosaccharide chains (glycans) covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated.

Grasshoppers are a group of insects belonging to the suborder Caelifera. They are among what is probably the most ancient living group of chewing herbivorous insects, dating back to the early Triassic around 250 million years ago.
FasII is initially expressed selectively localized to basolateral junctions during the process of oogenesis, where it functions to establish polarity in inner polar cells of epithelium-derived border cells. [2] During embryogenesis, fasII is dynamically expressed on a subset of axon fascicles in longitudinal nervous system pathways, [3] including the MP1 tract. [2] Here, fasII (and other attractive/repulsive environmental cues such as semaphorins and other morphogens) functions as a framework for pathfinding choices of newly extending axons. [2] This is achieved through trans-homophilic fasII-mediated adhesion and subsequent activation of downstream intracellular signaling pathways involving mitogen-activated protein kinase (MAPK) and regulation of intracellular calcium levels. [2] Later, fasII is expressed on growth cones of axons in other tracts including embryonic peripheral nervous system (PNS) motor neurons. [2] Only the transmembrane isoforms are expressed by neurons, while the GPI-linked form is expressed by non-neuronal cells (mainly glial cells), where it functions as a substrate for growth cones of extending axons, directing adhesion and axon guidance. [2] FasII is also expressed by clusters of differentiating neuroblasts at early stages of neurogenesis where its function is not fully understood but might be involved in proneural gene induction. [2]
Oogenesis, ovogenesis, or oögenesis is the differentiation of the ovum into a cell competent to further develop when fertilized. It is developed from the primary oocyte by maturation. Oogenesis is initiated in the embryonic stage.
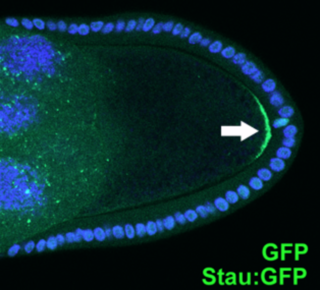
Cell polarity refers to spatial differences in shape, structure, and function within a cell. Almost all cell types exhibit some form of polarity, which enables them to carry out specialized functions. Classical examples of polarized cells are described below, including epithelial cells with apical-basal polarity, neurons in which signals propagate in one direction from dendrites to axons, and migrating cells. Furthermore, cell polarity is important during many types of asymmetric cell division to set up functional asymmetries between daughter cells.

Epithelium is one of the four basic types of animal tissue, along with connective tissue, muscle tissue and nervous tissue. Epithelial tissues line the outer surfaces of organs and blood vessels throughout the body, as well as the inner surfaces of cavities in many internal organs. An example is the epidermis, the outermost layer of the skin.
Other roles for fasII include delineating two axonal pathways in the adult ocellar sensory system (OSS) via its expression on ocellar pioneer (OP) neurons where it acts to promote neurite outgrowth from primary neurons (along with neuroglian) by activating fibroblast growth factor receptor (FGFR) signaling. [2] In addition, fasII has been shown to be involved in synaptic target selection, stabilization and remodeling along with several proteins such as netrins, semaphorins and other Ig-CAMs. [2]
A neurite or neuronal process refers to any projection from the cell body of a neuron. This projection can be either an axon or a dendrite. The term is frequently used when speaking of immature or developing neurons, especially of cells in culture, because it can be difficult to tell axons from dendrites before differentiation is complete.
The fibroblast growth factor receptors are, as their name implies, receptors that bind to members of the fibroblast growth factor family of proteins. Some of these receptors are involved in pathological conditions. For example, a point mutation in FGFR3 can lead to achondroplasia.
The human homolog is STAB2.