
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure C6H5CH2–. Benzyl features a benzene ring attached to a CH2 group.
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". In addition to pyrrolidine itself, many substituted pyrrolidines are known.

Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.

Macromerine is a phenethylamine derivative. It was first identified from the cactus Coryphantha macromeris. It can also be found in C. runyonii, C. elephantidens, and other related members of the family Cactaceae. The plants may have been used by Tarahumara shamans for their entheogenic effects.
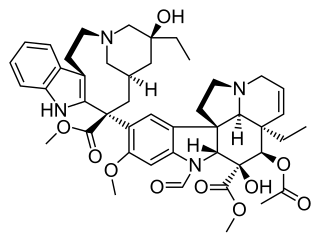
Vinca alkaloids are a set of anti-mitotic and anti-microtubule alkaloid agents originally derived from the periwinkle plant Catharanthus roseus and other vinca plants. They block beta-tubulin polymerization in a dividing cell.
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (c-c) in the process. A palladium (0) species is generally utilized as the metal catalyst, though nickel is sometimes used:

Dichroa febrifuga is a flowering plant in the family Hydrangeaceae. In the Indonesian language it is called gigil, alternatively tataruman in Sundanese, ramram in Papua, Hom dong (ฮอมดง) in Thai, but Yai khlang yai (ยายคลังใหญ่) in Nakhon Sri Thammarat, Yai Krang (ยายกรัง) in the South, and Hom Kham (ฮอมคำ) in Lanna.

The Gould–Jacobs reaction is an organic synthesis for the preparation of quinolines and 4‐hydroxyquinoline derivatives. The Gould-Jacobs reaction is a series of reactions. The series of reactions begins with the condensation/substitution of an aniline with alkoxy methylenemalonic ester or acyl malonic ester, producing anilidomethylenemalonic ester. Then through a 6 electron cyclization process, 4-hydroxy-3-carboalkoxyquinoline is formed, which exist mostly in the 4-oxo form. Saponification results in the formation of an acid. This step is followed by decarboxylation to give 4-hydroxyquinoline. The Gould-Jacobs reaction is effective for anilines with electron‐donating groups at the meta‐position.
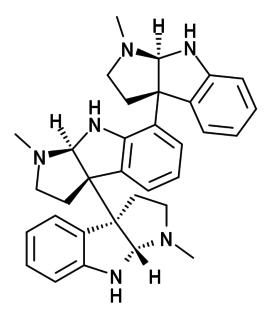
Hodgkinsine is an alkaloid found in plants of the genus Psychotria, particularly Psychotria colorata, although it is also found in Psychotria lyciiflora and probably other species in this family,

Horsfiline is an oxindole alkaloid found in the plant Horsfieldia superba, which is used in traditional herbal medicine. It has analgesic effects and has been the subject of research both to produce it synthetically by convenient routes and to develop analogues and derivatives which may have improved analgesic effects.
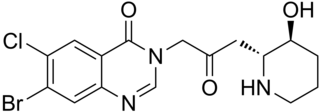
Halofuginone, sold under the brand name Halocur, is a coccidiostat used in veterinary medicine. It is a synthetic halogenated derivative of febrifugine, a natural quinazolinone alkaloid which can be found in the Chinese herb Dichroa febrifuga. Collgard Biopharmaceuticals is developing halofuginone for the treatment of scleroderma and it has received orphan drug designation from the U.S. Food and Drug Administration.
Gary H. Posner was Scowe Professor of Chemistry at Johns Hopkins University in Baltimore, Maryland. Posner is known for his pioneering research in organocopper chemistry, including his involvement in the development of the Corey-House-Posner-Whitesides reaction.

Indole is an aromatic heterocyclic organic compound with formula C8H7N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin.
Project 523 is a code name for a 1967 secret military project of the People's Republic of China to find antimalarial medications. Named after the date the project launched, 23 May, it addressed malaria, an important threat in the Vietnam War. At the behest of Ho Chi Minh, Prime Minister of North Vietnam, Zhou Enlai, the Premier of the People's Republic of China, convinced Mao Zedong, Chairman of the Communist Party of China, to start the mass project "to keep [the] allies' troops combat-ready", as the meeting minutes put it. More than 500 Chinese scientists were recruited. The project was divided into three streams. The one for investigating traditional Chinese medicine discovered and led to the development of a class of new antimalarial drugs called artemisinins. Launched during and lasting throughout the Cultural Revolution, Project 523 was officially terminated in 1981.

Conolidine is an indole alkaloid. Preliminary reports suggest that it could provide analgesic effects with few of the detrimental side-effects associated with opioids such as morphine, though at present it has only been evaluated in mouse models.

Synthesis of morphine-like alkaloids in chemistry describes the total synthesis of the natural morphinan class of alkaloids that includes codeine, morphine, oripavine, and thebaine and the closely related semisynthetic analogs methorphan, buprenorphine, hydromorphone, hydrocodone, isocodeine, naltrexone, nalbuphine, oxymorphone, oxycodone, and naloxone.
N-Sulfinyl imines are a class of imines bearing a sulfinyl group attached to nitrogen. These imines display usefully stereoselectivity reactivity and due to the presence of the chiral electron withdrawing N-sulfinyl group. They allow 1,2-addition of organometallic reagents to imines. The N-sulfinyl group exerts powerful and predictable stereodirecting effects resulting in high levels of asymmetric induction. Racemization of the newly created carbon-nitrogen stereo center is prevented because anions are stabilized at nitrogen. The sulfinyl chiral auxiliary is readily removed by simple acid hydrolysis. The addition of organometallic reagents to N-sulfinyl imines is the most reliable and versatile method for the asymmetric synthesis of amine derivatives. These building blocks have been employed in the asymmetric synthesis of numerous biologically active compounds.

2-Carbomethoxytropinone (2-CMT) is a commonly used organic intermediate in the synthesis of cocaine and its analogues. As of at least 1999 no reaction pathway has been discovered that synthesizes cocaine-like compounds without utilizing the reduction of 2-CMT. The structure of cocaine was discovered by Richard Willstätter in 1898 after he synthesized it from 2-carbomethoxytropinone. Although it was originally believed that 2-CMT in nature was ultimately derived from ornithine and acetic acid, subsequent research has indicated other pathways exist for the biosynthesis of 2-CMT. A β-keto ester, 2-CMT exists in equilibrium with its keto–enol tautomer.
Biomimetic synthesis is an area of organic chemical synthesis that is specifically biologically inspired. The term encompasses both the testing of a "biogenetic hypothesis" through execution of a series of reactions designed to parallel the proposed biosynthesis, as well as programs of study where a synthetic reaction or reactions aimed at a desired synthetic goal are designed to mimic one or more known enzymic transformations of an established biosynthetic pathway. The earliest generally cited example of a biomimetic synthesis is Sir Robert Robinson's organic synthesis of the alkaloid tropinone.

Bis(cyclopentadienyl)titanium(III) chloride, also known as the Nugent–RajanBabu reagent, is the organotitanium compound which exists as a dimer with the formula [(C5H5)2TiCl]2. It is an air sensitive green solid. The complex finds specialized use in synthetic organic chemistry as a single electron reductant.














