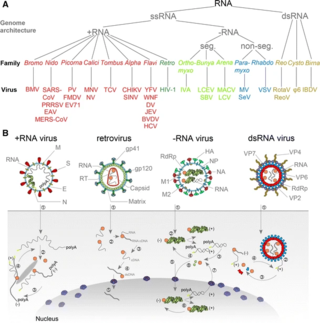
An RNA virus is a virus—other than a retrovirus—that has ribonucleic acid (RNA) as its genetic material. The nucleic acid is usually single-stranded RNA (ssRNA) but it may be double-stranded (dsRNA). Notable human diseases caused by RNA viruses include the common cold, influenza, SARS, MERS, COVID-19, Dengue Virus, hepatitis C, hepatitis E, West Nile fever, Ebola virus disease, rabies, polio, mumps, and measles.

Flaviviridae is a family of enveloped positive-strand RNA viruses which mainly infect mammals and birds. They are primarily spread through arthropod vectors. The family gets its name from the yellow fever virus; flavus is Latin for "yellow", and yellow fever in turn was named because of its propensity to cause jaundice in victims. There are 89 species in the family divided among four genera. Diseases associated with the group include: hepatitis (hepaciviruses), hemorrhagic syndromes, fatal mucosal disease (pestiviruses), hemorrhagic fever, encephalitis, and the birth defect microcephaly (flaviviruses).
Virus classification is the process of naming viruses and placing them into a taxonomic system similar to the classification systems used for cellular organisms.

Sedoreoviridae is a family of double-stranded RNA viruses. Member viruses have a wide host range, including vertebrates, invertebrates, plants, protists and fungi. They lack lipid envelopes and package their segmented genome within multi-layered capsids. Lack of a lipid envelope has allowed three-dimensional structures of these large complex viruses to be obtained, revealing a structural and likely evolutionary relationship to the cystovirus family of bacteriophage. There are currently 97 species in this family, divided among 15 genera in two subfamilies. Reoviruses can affect the gastrointestinal system and respiratory tract. The name "reo-" is an acronym for "respiratory enteric orphan" viruses. The term "orphan virus" refers to the fact that some of these viruses have been observed not associated with any known disease. Even though viruses in the family Reoviridae have more recently been identified with various diseases, the original name is still used.
A satellite is a subviral agent that depends on the coinfection of a host cell with a helper virus for its replication. Satellites can be divided into two major classes: satellite viruses and satellite nucleic acids. Satellite viruses, which are most commonly associated with plants, are also found in mammals, arthropods, and bacteria. They encode structural proteins to enclose their genetic material, which are therefore distinct from the structural proteins of their helper viruses. Satellite nucleic acids, in contrast, do not encode their own structural proteins, but instead are encapsulated by proteins encoded by their helper viruses. The genomes of satellites range upward from 359 nucleotides in length for satellite tobacco ringspot virus RNA (STobRV).

Plant viruses are viruses that affect plants. Like all other viruses, plant viruses are obligate intracellular parasites that do not have the molecular machinery to replicate without a host. Plant viruses can be pathogenic to vascular plants.

Geminiviridae is a family of plant viruses that encode their genetic information on a circular genome of single-stranded (ss) DNA. There are 520 species in this family, assigned to 14 genera. Diseases associated with this family include: bright yellow mosaic, yellow mosaic, yellow mottle, leaf curling, stunting, streaks, reduced yields. They have single-stranded circular DNA genomes encoding genes that diverge in both directions from a virion strand origin of replication. According to the Baltimore classification they are considered class II viruses. It is the largest known family of single stranded DNA viruses.

Nanoviridae is a family of viruses. Plants serve as natural hosts. There are currently 12 species in this family, divided among 2 genera and one unassigned species. Diseases associated with this family include: stunting. Their name is derived from the Greek word νᾶνος, because of their small genome and their stunting effect on infected plants.

Bromoviridae is a family of viruses. Plants serve as natural hosts. There are six genera in the family.
Closteroviridae is a family of viruses. Plants serve as natural hosts. There are four genera and 59 species in this family, seven of which are unassigned to a genus. Diseases associated with this family include: yellowing and necrosis, particularly affecting the phloem.

Tombusviridae is a family of single-stranded positive sense RNA plant viruses. There are three subfamilies, 17 genera, and 95 species in this family. The name is derived from Tomato bushy stunt virus (TBSV).

Tobamovirus is a genus of positive-strand RNA viruses in the family Virgaviridae. Many plants, including tobacco, potato, tomato, and squash, serve as natural hosts. Diseases associated with this genus include: necrotic lesions on leaves. The name Tobamovirus comes from the host and symptoms of the first virus discovered.
Idaeovirus is a genus of positive-sense ssRNA viruses that contains two species: Raspberry bushy dwarf virus (RBDV) and Privet idaeovirus. RBDV has two host-dependent clades: one for raspberries; the other for grapevines. Infections are a significant agricultural burden, resulting in decreased yield and quality of crops. RBDV has a synergistic relation with Raspberry leaf mottle virus, with co-infection greatly amplifying the concentration of virions in infected plants. The virus is transmitted via pollination with RBDV-infected pollen grains that first infect the stigma before causing systemic infection.

Sobemovirus is a genus of non-enveloped, positive-strand RNA viruses which infect plants.. Plants serve as natural hosts. There are 21 species in this genus. Diseases associated with this genus include: mosaics and mottles.
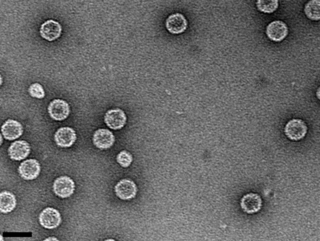
Picobirnavirus is a genus of double-stranded RNA viruses. It is the only genus in the family Picobirnaviridae. Although amniotes, especially mammals, were thought to serve as hosts, it has been recently suggested that these viruses might infect bacteria and possibly some other invertebrates. If they do infect bacteria, then they are Bacteriophages. There are three species in this genus. Associated symptoms include gastroenteritis in animals and humans, though the disease association is unclear.
Plectrovirus is a genus of viruses, in the family Plectroviridae. Bacteria in the phylum Mycoplasmatota serve as natural hosts, making these viruses bacteriophages. Acholeplasma virus L51 is the only species in the genus.
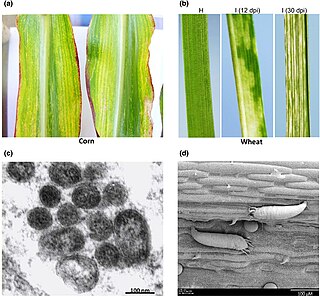
High Plains wheat mosaic emaravirus (WMoV), or High Plains virus (HPV) or Maize red stripe virus (MRSV/MRStV) is the causative agent of High plains disease of maize and wheat. It is spread by wheat curl mite, Aceria tosichella, which also transmits Wheat streak mosaic virus. The mite's ability to transmit a number of different viruses to cereal crops make it an economically important agricultural pest. In late June 2017 this virus was first detected in Canada, in Alberta. The Alberta samples were 99% similar to those in the USA. As Wheat streak mosaic virus is already present in Alberta, and coinfection with these two causes even more severe damage, this could cause much higher yield losses.

Emaravirus is a genus of negative-strand RNA viruses which infect plants. The plant virus group is the sole genus in the family Fimoviridae. The genus has 21 species.
Lolavirus is a genus of viruses in the order Tymovirales, in the family Alphaflexiviridae. Plants, specifically ryegrass, serve as natural hosts. There is only one species in this genus: Lolium latent virus.

Positive-strand RNA viruses are a group of related viruses that have positive-sense, single-stranded genomes made of ribonucleic acid. The positive-sense genome can act as messenger RNA (mRNA) and can be directly translated into viral proteins by the host cell's ribosomes. Positive-strand RNA viruses encode an RNA-dependent RNA polymerase (RdRp) which is used during replication of the genome to synthesize a negative-sense antigenome that is then used as a template to create a new positive-sense viral genome.
















