
The enzyme-linked immunosorbent assay (ELISA) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand in a liquid sample using antibodies directed against the ligand to be measured. ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries.

A fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.

Immunofluorescence(IF) is a light microscopy-based technique that allows detection and localization of a wide variety of target biomolecules within a cell or tissue at a quantitative level. The technique utilizes the binding specificity of antibodies and antigens. The specific region an antibody recognizes on an antigen is called an epitope. Several antibodies can recognize the same epitope but differ in their binding affinity. The antibody with the higher affinity for a specific epitope will surpass antibodies with a lower affinity for the same epitope.

In molecular biology, biochips are engineered substrates that can host large numbers of simultaneous biochemical reactions. One of the goals of biochip technology is to efficiently screen large numbers of biological analytes, with potential applications ranging from disease diagnosis to detection of bioterrorism agents. For example, digital microfluidic biochips are under investigation for applications in biomedical fields. In a digital microfluidic biochip, a group of (adjacent) cells in the microfluidic array can be configured to work as storage, functional operations, as well as for transporting fluid droplets dynamically.
Plate readers, also known as microplate readers or microplate photometers, are instruments which are used to detect biological, chemical or physical events of samples in microtiter plates. They are widely used in research, drug discovery, bioassay validation, quality control and manufacturing processes in the pharmaceutical and biotechnological industry and academic organizations. Sample reactions can be assayed in 1-1536 well format microtiter plates. The most common microplate format used in academic research laboratories or clinical diagnostic laboratories is 96-well with a typical reaction volume between 100 and 200 μL per well. Higher density microplates are typically used for screening applications, when throughput and assay cost per sample become critical parameters, with a typical assay volume between 5 and 50 μL per well. Common detection modes for microplate assays are absorbance, fluorescence intensity, luminescence, time-resolved fluorescence, and fluorescence polarization.

High-throughput screening (HTS) is a method for scientific discovery especially used in drug discovery and relevant to the fields of biology, materials science and chemistry. Using robotics, data processing/control software, liquid handling devices, and sensitive detectors, high-throughput screening allows a researcher to quickly conduct millions of chemical, genetic, or pharmacological tests. Through this process one can quickly recognize active compounds, antibodies, or genes that modulate a particular biomolecular pathway. The results of these experiments provide starting points for drug design and for understanding the noninteraction or role of a particular location.
An immunoassay (IA) is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody (usually) or an antigen (sometimes). The molecule detected by the immunoassay is often referred to as an "analyte" and is in many cases a protein, although it may be other kinds of molecules, of different sizes and types, as long as the proper antibodies that have the required properties for the assay are developed. Analytes in biological liquids such as serum or urine are frequently measured using immunoassays for medical and research purposes.
A protein microarray is a high-throughput method used to track the interactions and activities of proteins, and to determine their function, and determining function on a large scale. Its main advantage lies in the fact that large numbers of proteins can be tracked in parallel. The chip consists of a support surface such as a glass slide, nitrocellulose membrane, bead, or microtitre plate, to which an array of capture proteins is bound. Probe molecules, typically labeled with a fluorescent dye, are added to the array. Any reaction between the probe and the immobilised protein emits a fluorescent signal that is read by a laser scanner. Protein microarrays are rapid, automated, economical, and highly sensitive, consuming small quantities of samples and reagents. The concept and methodology of protein microarrays was first introduced and illustrated in antibody microarrays in 1983 in a scientific publication and a series of patents. The high-throughput technology behind the protein microarray was relatively easy to develop since it is based on the technology developed for DNA microarrays, which have become the most widely used microarrays.

Texas Red or sulforhodamine 101 acid chloride is a red fluorescent dye, used in histology for staining cell specimens, for sorting cells with fluorescent-activated cell sorting machines, in fluorescence microscopy applications, and in immunohistochemistry. Texas Red fluoresces at about 615 nm, and the peak of its absorption spectrum is at 589 nm. The powder is dark purple. Solutions can be excited by a dye laser tuned to 595-605 nm, or less efficiently a krypton laser at 567 nm. The absorption extinction coefficient at 596 nm is about 85,000 M−1cm−1.
Salicylate testing is a category of drug testing that is focused on detecting salicylates such as acetysalicylic acid for either biochemical or medical purposes.

Immunocytochemistry (ICC) is a common laboratory technique that is used to anatomically visualize the localization of a specific protein or antigen in cells by use of a specific primary antibody that binds to it. The primary antibody allows visualization of the protein under a fluorescence microscope when it is bound by a secondary antibody that has a conjugated fluorophore. ICC allows researchers to evaluate whether or not cells in a particular sample express the antigen in question. In cases where an immunopositive signal is found, ICC also allows researchers to determine which sub-cellular compartments are expressing the antigen.

An antibody microarray is a specific form of protein microarray. In this technology, a collection of captured antibodies are spotted and fixed on a solid surface such as glass, plastic, membrane, or silicon chip, and the interaction between the antibody and its target antigen is detected. Antibody microarrays are often used for detecting protein expression from various biofluids including serum, plasma and cell or tissue lysates. Antibody arrays may be used for both basic research and medical and diagnostic applications.
Fluorescence anisotropy or fluorescence polarization is the phenomenon where the light emitted by a fluorophore has unequal intensities along different axes of polarization. Early pioneers in the field include Aleksander Jablonski, Gregorio Weber, and Andreas Albrecht. The principles of fluorescence polarization and some applications of the method are presented in Lakowicz's book.

A lateral flow test (LFT), is an assay also known as a lateral flow device (LFD), lateral flow immunochromatographic assay, or rapid test. It is a simple device intended to detect the presence of a target substance in a liquid sample without the need for specialized and costly equipment. LFTs are widely used in medical diagnostics in the home, at the point of care, and in the laboratory. For instance, the home pregnancy test is an LFT that detects a specific hormone. These tests are simple and economical and generally show results in around five to thirty minutes. Many lab-based applications increase the sensitivity of simple LFTs by employing additional dedicated equipment. Because the target substance is often a biological antigen, many lateral flow tests are rapid antigen tests.

Fluorescence is used in the life sciences generally as a non-destructive way of tracking or analysing biological molecules. Some proteins or small molecules in cells are naturally fluorescent, which is called intrinsic fluorescence or autofluorescence. Alternatively, specific or general proteins, nucleic acids, lipids or small molecules can be "labelled" with an extrinsic fluorophore, a fluorescent dye which can be a small molecule, protein or quantum dot. Several techniques exist to exploit additional properties of fluorophores, such as fluorescence resonance energy transfer, where the energy is passed non-radiatively to a particular neighbouring dye, allowing proximity or protein activation to be detected; another is the change in properties, such as intensity, of certain dyes depending on their environment allowing their use in structural studies.
Mass spectrometric immunoassay (MSIA) is a rapid method is used to detect and/ or quantify antigens and or antibody analytes. This method uses an analyte affinity isolation to extract targeted molecules and internal standards from biological fluid in preparation for matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS). This method allows for "top down" and "bottom up" analysis. This sensitive method allows for a new and improved process for detecting multiple antigens and antibodies in a single assay. This assay is also capable of distinguishing mass shifted forms of the same molecule via a panantibody, as well as distinguish point mutations in proteins. Each specific form is detected uniquely based on their characteristic molecular mass. MSIA has dual specificity because of the antibody-antigen reaction coupled with the power of a mass spectrometer.
Time-resolved fluorescence energy transfer (TR-FRET) is the practical combination of time-resolved fluorometry (TRF) with Förster resonance energy transfer (FRET) that offers a powerful tool for drug discovery researchers. TR-FRET combines the low background aspect of TRF with the homogeneous assay format of FRET. The resulting assay provides an increase in flexibility, reliability and sensitivity in addition to higher throughput and fewer false positive/false negative results. FRET involves two fluorophores, a donor and an acceptor. Excitation of the donor by an energy source produces an energy transfer to the acceptor if the two are within a given proximity to each other. The acceptor in turn emits light at its characteristic wavelength.
A thermal shift assay (TSA) measures changes in the thermal denaturation temperature and hence stability of a protein under varying conditions such as variations in drug concentration, buffer pH or ionic strength, redox potential, or sequence mutation. The most common method for measuring protein thermal shifts is differential scanning fluorimetry (DSF) or thermofluor, which utilizes specialized fluorogenic dyes.
A ligand binding assay (LBA) is an assay, or an analytic procedure, which relies on the binding of ligand molecules to receptors, antibodies or other macromolecules. A detection method is used to determine the presence and extent of the ligand-receptor complexes formed, and this is usually determined electrochemically or through a fluorescence detection method. This type of analytic test can be used to test for the presence of target molecules in a sample that are known to bind to the receptor.
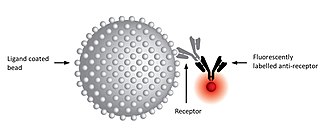
A kinetic exclusion assay (KinExA) is a type of bioassay in which a solution containing receptor, ligand, and receptor-ligand complex is briefly exposed to additional ligand immobilized on a solid phase.














