
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene and is a PFAS that has numerous applications. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from DuPont, which originally discovered the compound in 1938.

The Cape Fear River is a 191.08-mile-long blackwater river in east-central North Carolina. It flows into the Atlantic Ocean near Cape Fear, from which it takes its name. The river is formed at the confluence of the Haw River and the Deep River in the town of Moncure, North Carolina. Its river basin is the largest in the state: 9,149 sq mi.

Perfluorooctanoic acid is a perfluorinated carboxylic acid produced and used worldwide as an industrial surfactant in chemical processes and as a material feedstock. PFOA is considered a surfactant, or fluorosurfactant, due to its chemical structure, which consists of a perfluorinated, n-heptyl "tail group" and a carboxylate "head group". The head group can be described as hydrophilic while the fluorocarbon tail is both hydrophobic and lipophobic.

Perfluorooctanesulfonic acid (PFOS) is a chemical compound having an eight-carbon fluorocarbon chain and a sulfonic acid functional group and thus a perfluorosulfonic acid. It is an anthropogenic (man-made) fluorosurfactant, now regarded as a global pollutant. PFOS was the key ingredient in Scotchgard, a fabric protector made by 3M, and related stain repellents. The acronym "PFOS" refers to the parent sulfonic acid and to various salts of perfluorooctanesulfonate. These are all colorless or white, water-soluble solids. Although of low acute toxicity, PFOS has attracted much attention for its pervasiveness and environmental impact. It was added to Annex B of the Stockholm Convention on Persistent Organic Pollutants in May 2009.

Biosolids are solid organic matter recovered from a sewage treatment process and used as fertilizer. In the past, it was common for farmers to use animal manure to improve their soil fertility. In the 1920s, the farming community began also to use sewage sludge from local wastewater treatment plants. Scientific research over many years has confirmed that these biosolids contain similar nutrients to those in animal manures. Biosolids that are used as fertilizer in farming are usually treated to help to prevent disease-causing pathogens from spreading to the public. Some sewage sludge can not qualify as biosolids due to persistent, bioaccumulative and toxic chemicals, radionuclides, and heavy metals at levels sufficient to contaminate soil and water when applied to land.

Persistent organic pollutants (POPs) are organic compounds that are resistant to degradation through chemical, biological, and photolytic processes. They are toxic chemicals that adversely affect human health and the environment around the world. Because they can be transported by wind and water, most POPs generated in one country can and do affect people and wildlife far from where they are used and released.

Solvay is a Belgian multinational chemical company established in 1863, with its headquarters located in Neder-Over-Heembeek, Brussels, Belgium.

Environmental toxicology is a multidisciplinary field of science concerned with the study of the harmful effects of various chemical, biological and physical agents on living organisms. Ecotoxicology is a subdiscipline of environmental toxicology concerned with studying the harmful effects of toxicants at the population and ecosystem levels.
Perfluorononanoic acid, or PFNA, is a synthetic perfluorinated carboxylic acid and fluorosurfactant that is also an environmental contaminant found in people and wildlife along with PFOS and PFOA.

Per- and polyfluoroalkyl substances (PFAS or PFASs) are a group of synthetic organofluorine chemical compounds that have multiple fluorine atoms attached to an alkyl chain. An early definition, from 2011, required that they contain at least one perfluoroalkyl moiety, –CnF2n+1–. Beginning in 2021, the Organisation for Economic Co-operation and Development (OECD) expanded their terminology, stating that "PFASs are defined as fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/I atom attached to it), i.e., with a few noted exceptions, any chemical with at least a perfluorinated methyl group (–CF3) or a perfluorinated methylene group (–CF2–) is a PFAS."

Perfluorobutanesulfonic acid (PFBS) is a PFAS chemical compound having a four-carbon fluorocarbon chain and a sulfonic acid functional group. It is stable and unreactive because of the strength of carbon–fluorine bonds. It can occur in the form of a colorless liquid or a corrosive solid. Its conjugate base is perfluorobutanesulfonate which functions as the hydrophobe in fluorosurfactants.
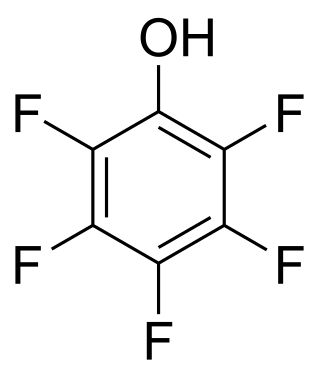
A perfluorinated compound (PFC) or perfluoro compound is an organofluorine compound lacking C-H bonds. Many perfluorinated compounds have properties that are quite different from their C-H containing analogues. Common functional groups in PFCs are OH, CO2H, chlorine, O, and SO3H. Electrofluorination is the predominant method for their production. Due to their chemical stability, some of these perfluorinated compounds bioaccumulate.

Perfluoroalkoxy alkanes (PFA) are fluoropolymers. They are copolymers of tetrafluoroethylene (C2F4) and perfluoroethers (C2F3ORf, where Rf is a perfluorinated group such as trifluoromethyl (CF3)). The properties of these polymers are similar to those of polytetrafluoroethylene (PTFE). Compared to PTFE, PFA has better anti-stick properties and higher chemical resistance, at the expense of lesser scratch resistance.
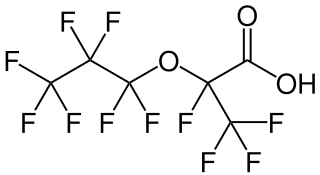
FRD-903 is a chemical compound that is among the class of per- and polyfluoroalkyl substances (PFASs). More specifically, this synthetic petrochemical is also described as a perfluoroalkyl ether carboxylic acid (PFECA) and a Fluorointermediate. It is not biodegradable and is not hydrolyzed by water.
GenX is a Chemours trademark name for a synthetic, short-chain organofluorine chemical compound, the ammonium salt of hexafluoropropylene oxide dimer acid (HFPO-DA). It can also be used more informally to refer to the group of related fluorochemicals that are used to produce GenX. DuPont began the commercial development of GenX in 2009 as a replacement for perfluorooctanoic acid.

The CompTox Chemicals Dashboard is a freely accessible online database created and maintained by the U.S. Environmental Protection Agency (EPA). The database provides access to multiple types of data including physicochemical properties, environmental fate and transport, exposure, usage, in vivo toxicity, and in vitro bioassay. EPA and other scientists use the data and models contained within the dashboard to help identify chemicals that require further testing and reduce the use of animals in chemical testing. The Dashboard is also used to provide public access to information from EPA Action Plans, e.g. around perfluorinated alkylated substances.
This timeline of events related to per- and polyfluoroalkyl substances (PFASs) includes events related to the discovery, development, manufacture, marketing, uses, concerns, litigation, regulation, and legislation, involving the human-made PFASs. The timeline focuses on some perfluorinated compounds, particularly perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) and on the companies that manufactured and marketed them, mainly DuPont and 3M. An example of PFAS is the fluorinated polymer polytetrafluoroethylene (PTFE), which has been produced and marketed by DuPont under its trademark Teflon. GenX chemicals and perfluorobutanesulfonic acid (PFBS) are organofluorine chemicals used as a replacement for PFOA and PFOS.
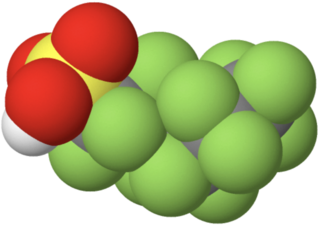
Perfluorohexanesulfonic acid (PFHxS) is a synthetic chemical compound. It is one of many compounds collectively known as per- and polyfluoroalkyl substances (PFASs). It is an anionic fluorosurfactant and a persistent organic pollutant with bioaccumulative properties. Although the use of products containing PFHxS and other PFASs have been banned or are being phased out in many jurisdictions, it remains ubiquitous in many environments and within the general population, and is one of the most commonly detected PFASs.
From the 1950s through the early 2000s, 3M disposed of per- and polyfluoroalkyl substances produced during the manufacturing process of various industrial products in four dumping sites in Minnesota. These chemicals have contaminated the groundwater of over 170,000 residents of the Twin Cities East Metro Area, culminating in an $850 million settlement with the State of Minnesota in 2018.

















