
Xanthoria parietina is a foliose lichen in the family Teloschistaceae. It has wide distribution, and many common names such as common orange lichen, yellow scale, maritime sunburst lichen and shore lichen. It can be found near the shore on rocks or walls, and also on inland rocks, walls, or tree bark. It was chosen as a model organism for genomic sequencing by the US Department of Energy Joint Genome Institute (JGI).

Copaene, or more precisely, α-copaene, is the common chemical name of an oily liquid hydrocarbon that is found in a number of essential oil-producing plants. The name is derived from that of the resin-producing tropical copaiba tree, Copaifera langsdorffii, from which the compound was first isolated in 1914. Its structure, including the chirality, was determined in 1963. The double-bond isomer with an exocyclic-methylene group, β-copaene, was first reported in 1967.

Parietin is the predominant cortical pigment of lichens in the genus Caloplaca, a secondary product of the lichen Xanthoria parietina, and a pigment found in the roots of curled dock. It has an orange-yellow color and absorbs blue light.

The Teloschistaceae are a large family of mostly lichen-forming fungi belonging to the class Lecanoromycetes in the division Ascomycota. The family has a cosmopolitan distribution, although its members occur predominantly in temperate regions. Most members are lichens that either live on rock or on bark, but about 40 species are lichenicolous – meaning they are non-lichenised fungi that live on other lichens. Many members of the Teloschistaceae are readily identifiable by their vibrant orange to yellow hue, a result of their frequent anthraquinone content. The presence of these anthraquinone pigments, which confer protection from ultraviolet light, enabled this group to expand from shaded forest habitats to harsher environmental conditions of sunny and arid ecosystems during the Late Cretaceous.
Iron phosphide is a chemical compound of iron and phosphorus, with a formula of FeP.< Its physical appearance is grey needles.
Tellurium iodide is an inorganic compound with the formula TeI. Two forms are known. Their structures differ from the other monohalides of tellurium. There are three subiodides of tellurium, α-TeI, β-TeI, and Te2I, and one tellurium tetraiodide.
Arne Haaland was a Norwegian chemist.
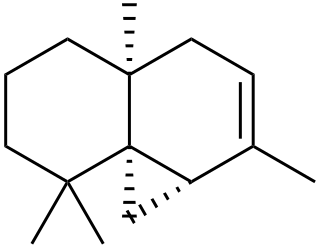
Thujopsene is a natural chemical compound, classified as a sesquiterpene, with the molecular formula C15H24.

Thiomuscimol is a GABAA receptor agonist which is structurally related to muscimol.

Silver sulfite is the chemical compound with the formula Ag2SO3. This unstable silver compound when heated and/or in light it decomposes to silver dithionate and silver sulfate.
A spot test in lichenology is a spot analysis used to help identify lichens. It is performed by placing a drop of a chemical reagent on different parts of the lichen and noting the colour change associated with application of the chemical. The tests are routinely encountered in dichotomous keys for lichen species, and they take advantage of the wide array of lichen products produced by lichens and their uniqueness among taxa. As such, spot tests reveal the presence or absence of chemicals in various parts of a lichen. They were first proposed as a method to help identify species by the Finnish lichenologist William Nylander in 1866.
Streptomyces armeniacus is a bacterium species from the genus Streptomyces which has isolated from soil. Streptomyces armeniacus produces streptopyrrole.

Palladium(II) sulfide is a chemical compound of palladium and sulfur with the chemical formula PdS. Like other palladium and platinum chalcogenides, palladium(II) sulfide has complex structural, electrical and magnetic properties.

Strepsilin is a chemical found in lichens. It produces an emerald green colour in the C test. It is a dibenzofuran dimer, with hydroxy, oxy and methyl side groups. It is named after Cladonia strepsilis. Strepsilin was discovered by Wilhelm Zopf in 1903. The structure of strepsilin was determined by Shoji Shibata.
Antimony(III) oxide hydroxide nitrate is an inorganic compound with the chemical formula Sb4O4(OH)2(NO3)2. It is one of the very few nitrates of antimony. No evidence for a simple trinitrate has been reported. According to X-ray crystallography, its structure consists of cationic layers of antimony oxide/hydroxide with intercalated nitrate anions. This compound is produced by the reaction of antimony(III) oxide and nitric acid at 110 °C.
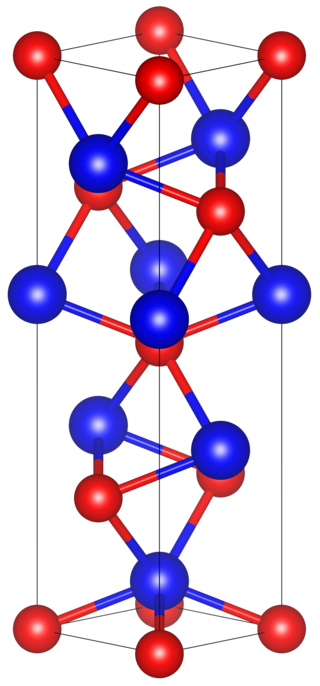
Tantalum arsenide is a compound of tantalum and arsenic with the formula TaAs. It is notable as being the first topological Weyl semimetal that was identified and characterized by ARPES.

Kuettlingeria soralifera is a saxicolous (rock-dwelling), crustose lichen species in the family Teloschistaceae, first described in 2006. It is similar to Kuettlingeria xerica but distinguished by the presence of soredia on its thallus.

Parietinic acid is an organic compound in the structural class of chemicals known as anthraquinones. It is found in many species of the lichen family Teloschistaceae. The substance was first reported in the literature by the German chemist Walter Eschrich in 1958.
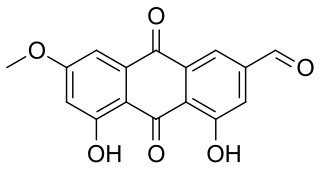
Fallacinal is an organic compound in the structural class of chemicals known as anthraquinones. It is found in many species of the lichen family Teloschistaceae.

Fallacinol (teloschistin) is an organic compound in the structural class of chemicals known as anthraquinones. It is found in some lichens, particularly in the family Teloschistaceae, as well as a couple of plants and non lichen-forming fungi. In 1936, Japanese chemists isolated a pigment they named fallacin from the lichen Oxneria fallax, which was later refined and assigned a tentative structural formula; by 1949, Indian chemists had isolated a substance from Teloschistes flavicans with an identical structural formula to fallacin. Later research further separated fallacin into two distinct pigments, fallacin-A and fallacin-B (fallacinol). The latter compound is also known as teloschistin due to its structural match with the substance isolated earlier.














