Classical free particle
The classical free particle is characterized by a fixed velocity v. The momentum of a particle with mass m is given by and the kinetic energy (equal to total energy) by .
In physics, a free particle is a particle that, in some sense, is not bound by an external force, or equivalently not in a region where its potential energy varies. In classical physics, this means the particle is present in a "field-free" space. In quantum mechanics, it means the particle is in a region of uniform potential, usually set to zero in the region of interest since the potential can be arbitrarily set to zero at any point in space.
The classical free particle is characterized by a fixed velocity v. The momentum of a particle with mass m is given by and the kinetic energy (equal to total energy) by .
A free particle with mass in non-relativistic quantum mechanics is described by the free Schrödinger equation:
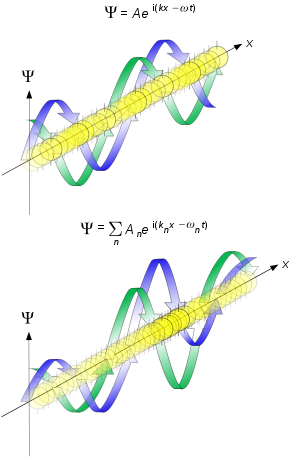
where ψ is the wavefunction of the particle at position r and time t. The solution for a particle with momentum p or wave vector k, at angular frequency ω or energy E, is given by a complex plane wave:
with amplitude A and has two different rules according to its mass:
The eigenvalue spectrum is infinitely degenerate since for each eigenvalue E>0, there corresponds an infinite number of eigenfunctions corresponding to different directions of .
The De Broglie relations: , apply. Since the potential energy is (stated to be) zero, the total energy E is equal to the kinetic energy, which has the same form as in classical physics:
As for all quantum particles free or bound, the Heisenberg uncertainty principles apply. It is clear that since the plane wave has definite momentum (definite energy), the probability of finding the particle's location is uniform and negligible all over the space. In other words, the wave function is not normalizable in a Euclidean space, these stationary states can not correspond to physical realizable states. [1] [2]
The normalization condition for the wave function states that if a wavefunction belongs to the quantum state space [3] then the integral of the probability density function
where * denotes complex conjugate, over all space is the probability of finding the particle in all space, which must be unity if the particle exists: The state of a free particle given by plane wave solutions is not normalizable as for any fixed time . Using wave packets, however, the states can be expressed as functions that are normalizable.
Using the Fourier inversion theorem, the free particle wave function may be represented by a superposition of momentum eigenfunctions, or, wave packet: [4] where and is the Fourier transform of a "sufficiently nice" initial wavefunction .
The expectation value of the momentum p for the complex plane wave is
and for the general wave packet it is
The expectation value of the energy E is

The phase velocity is defined to be the speed at which a plane wave solution propagates, namely
Note that is not the speed of a classical particle with momentum ; rather, it is half of the classical velocity.
Meanwhile, suppose that the initial wave function is a wave packet whose Fourier transform is concentrated near a particular wave vector . Then the group velocity of the plane wave is defined as
which agrees with the formula for the classical velocity of the particle. The group velocity is the (approximate) speed at which the whole wave packet propagates, while the phase velocity is the speed at which the individual peaks in the wave packet move. [5] The figure illustrates this phenomenon, with the individual peaks within the wave packet propagating at half the speed of the overall packet.
The notion of group velocity is based on a linear approximation to the dispersion relation near a particular value of . [6] In this approximation, the amplitude of the wave packet moves at a velocity equal to the group velocity without changing shape. This result is an approximation that fails to capture certain interesting aspects of the evolution a free quantum particle. Notably, the width of the wave packet, as measured by the uncertainty in the position, grows linearly in time for large times. This phenomenon is called the spread of the wave packet for a free particle.
Specifically, it is not difficult to compute an exact formula for the uncertainty as a function of time, where is the position operator. Working in one spatial dimension for simplicity, we have: [7] where is the time-zero wave function. The expression in parentheses in the second term on the right-hand side is the quantum covariance of and .
Thus, for large positive times, the uncertainty in grows linearly, with the coefficient of equal to . If the momentum of the initial wave function is highly localized, the wave packet will spread slowly and the group-velocity approximation will remain good for a long time. Intuitively, this result says that if the initial wave function has a very sharply defined momentum, then the particle has a sharply defined velocity and will (to good approximation) propagate at this velocity for a long time.
There are a number of equations describing relativistic particles: see relativistic wave equations.