
Neomycin is an aminoglycoside antibiotic that displays bactericidal activity against Gram-negative aerobic bacilli and some anaerobic bacilli where resistance has not yet arisen. It is generally not effective against Gram-positive bacilli and anaerobic Gram-negative bacilli. Neomycin comes in oral and topical formulations, including creams, ointments, and eyedrops. Neomycin belongs to the aminoglycoside class of antibiotics that contain two or more amino sugars connected by glycosidic bonds.

Streptomyces is the largest genus of Actinomycetota, and the type genus of the family Streptomycetaceae. Over 700 species of Streptomyces bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, and have very large genomes with high GC content. Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin. Different strains of the same species may colonize very diverse environments.

Geldanamycin is a 1,4-benzoquinone ansamycin antitumor antibiotic that inhibits the function of Hsp90 by binding to the unusual ADP/ATP-binding pocket of the protein. HSP90 client proteins play important roles in the regulation of the cell cycle, cell growth, cell survival, apoptosis, angiogenesis and oncogenesis.

Enediynes are organic compounds containing two triple bonds and one double bond.

Sparsomycin is a compound, initially discovered as a metabolite of the bacterium Streptomyces sparsogenes, which binds to the 50S ribosomal subunit and inhibits protein synthesis through peptidyl transferase inhibition. As it binds to the 50S ribosomal subunit, it induces translocation on the 30S subunit. It is a nucleotide analogue. It was also formerly thought to be a possible anti-tumor agent, but interest in this drug was later discarded after it was discovered that it resulted in retinopathy and as a tool to study protein synthesis; it is not specific for bacterial ribosomes and so not usable as an antibiotic.
Streptomyces aculeolatus is a bacterium species from the genus of Streptomyces which has been isolated from soil from the Tottori prefecture in Japan. Streptomyces aculeolatus produces naphthablin.
Streptomyces alanosinicus is a bacterium species from the genus of Streptomyces which was isolated from soil in Brazil. Streptomyces alanosinicus produces the antibiotics alanosine and spicamycin.
Streptomyces albofaciens is a bacterium species from the genus of Streptomyces which produces oxytetracycline, spiramycin, albopeptin A, albopeptin B and alpomycin.
Streptomyces canus is a bacterium species from the genus of Streptomyces which has been isolated from soil in the US. Streptomyces canus produces resistomycin, tetracenomycin D, amphomycin, aspartocin D and aspartocin E.
Streptomyces cinnamoneus is a bacterium species from the genus of Streptomyces which has been isolated from soil in Japan. Streptomyces cinnamoneus produces duramycin A, duramycin B, duramycin C, carbomycin, cinnomycin and fungichromin.
Streptomyces griseorubens is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces griseorubens produces althiomycin. Streptomyces griseorubens produces matamycin and althiomycin.
Streptomyces halstedii is a bacterium species from the genus of Streptomyces which has been isolated from deeper soil layers. Streptomyces halstedii produces magnamycin B, vicenistatin deltamycin A2, deltamycin A3, bafilomycin B1 and bafilomycin C1. Streptomyces halstedii also produces complex antifungal antibiotics like oligomycins and the antibiotics anisomycin and sinefungin.
Streptomyces microflavus is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces microflavus produces nemadectin, fattiviracin A1, milbemycin and deoxyuridines. Streptomyces microflavus also produces the ionophore valinomycin. Streptomyces microflavus is also known to cause potato common scab disease in Korea.
Streptomyces parvulus is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces parvulus produces the peptide antibiotic Actinomycin D and the angiogenesis inhibitor borrelidin and manumycin A, himalomycin A, himalomycin B and kynurenine.

Carbocyclic nucleosides are nucleoside analogues in which a methylene group has replaced the oxygen atom of the furanose ring. These analogues have the nucleobase attached at a simple alkyl carbon rather than being part of a hemiaminal ether linkage. As a result, they have increased chemical stability. They also have increased metabolic stability because they are unaffected by phosphorylases and hydrolases that cleave the glycosidic bond between the nucleobase and furanose ring of nucleosides. They retain many of the biological properties of the original nucleosides with respect to recognition by various enzymes and receptors.
Kitasatospora purpeofusca is a bacterium species from the genus Kitasatospora which has been isolated from soil in Japan. Kitasatospora purpeofusca produces negamycin, aestivophoenin A, aestivophoenin B, aestivophoenin C and heptaene.
Streptomyces rochei is a bacterium species from the genus of Streptomyces which has been isolated from soil in Russia. Streptomyces rochei produces borrelidin, butyrolactol A, butyrolactol B, uricase and streptothricin. Streptomyces rochei has antifungal activity against Fusarium oxysporum f.sp. lycopersici and Aspergillus fumigatus. Streptomyces rochei produces moenomycin and bambermycin. Streptomyces rochei produces amicetin A, amicetin B, amicetin C and streptolin. Streptomyces rochei produces endo-β-N-acetylglucosaminidase mithramycin, amicetin, bamicetin, and plicacetin.
Streptomyces roseofulvus is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces roseofulvus produces deoxyfrenolicin and frenolicin B.

C-1027 or lidamycin is an antitumor antibiotic consisting of a complex of an enediyne chromophore and an apoprotein. It shows antibiotic activity against most Gram-positive bacteria. It is one of the most potent cytotoxic molecules known, due to its induction of a higher ratio of DNA double-strand breaks than single-strand breaks.
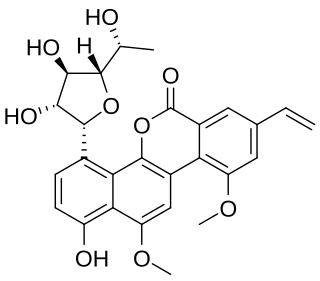
Gilvocarcin V is an antitumor agent and an antibiotic which is active against Gram-positive bacteria with the molecular formula C27H26O9. Gilvocarcin V is produced by the bacterium Streptomyces griseoflavus and other Streptomyces bacteria. Gilvocarcin V is a strong inhibitor of the DNA synthesis.








