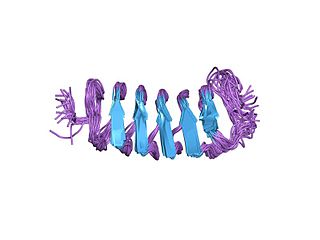
Antifreeze proteins (AFPs) or ice structuring proteins refer to a class of polypeptides produced by certain animals, plants, fungi and bacteria that permit their survival in temperatures below the freezing point of water. AFPs bind to small ice crystals to inhibit the growth and recrystallization of ice that would otherwise be fatal. There is also increasing evidence that AFPs interact with mammalian cell membranes to protect them from cold damage. This work suggests the involvement of AFPs in cold acclimatization.
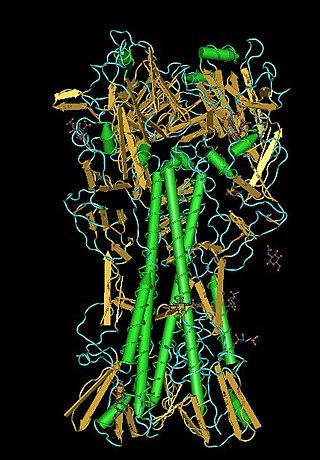
Lectins are carbohydrate-binding proteins that are highly specific for sugar groups that are part of other molecules, so cause agglutination of particular cells or precipitation of glycoconjugates and polysaccharides. Lectins have a role in recognition at the cellular and molecular level and play numerous roles in biological recognition phenomena involving cells, carbohydrates, and proteins. Lectins also mediate attachment and binding of bacteria, viruses, and fungi to their intended targets.
In biology and biochemistry, protease inhibitors, or antiproteases, are molecules that inhibit the function of proteases. Many naturally occurring protease inhibitors are proteins.

Mannan-binding lectin serine protease 1 also known as mannose-associated serine protease 1 (MASP-1) is an enzyme that in humans is encoded by the MASP1 gene.

Pore-forming proteins are usually produced by bacteria, and include a number of protein exotoxins but may also be produced by other organisms such as apple snails that produce perivitellin-2 or earthworms, who produce lysenin. They are frequently cytotoxic, as they create unregulated pores in the membrane of targeted cells.

Chemokine ligand (XCL1) is a small cytokine belonging to the C chemokine family that is also known as lymphotactin. Chemokines are known for their function in inflammatory and immunological responses. This family C chemokines differs in structure and function from most chemokines. There are only two chemokines in this family and what separated them from other chemokines is that they only have two cysteines; one N-terminal cysteine and one cysteine downstream. These both are called Lymphotactin, alpha and beta form, and claim special characteristics only found between the two. Lymphotactins can go through a reversible conformational change which changes its binding shifts.

C-type lectin domain family 7 member A or Dectin-1 is a protein that in humans is encoded by the CLEC7A gene. CLEC7A is a member of the C-type lectin/C-type lectin-like domain (CTL/CTLD) superfamily. The encoded glycoprotein is a small type II membrane receptor with an extracellular C-type lectin-like domain fold and a cytoplasmic domain with a partial immunoreceptor tyrosine-based activation motif. It functions as a pattern-recognition receptor for a variety of β-1,3-linked and β-1,6-linked glucans from fungi and plants, and in this way plays a role in innate immune response. Expression is found on myeloid dendritic cells, monocytes, macrophages and B cells. Alternate transcriptional splice variants, encoding different isoforms, have been characterized. This gene is closely linked to other CTL/CTLD superfamily members on chromosome 12p13 in the natural killer gene complex region.

Ficolin-2, which was initially identified as L-ficolin, is a protein that in humans is encoded by the FCN2 gene.

C-type lectin domain family 4 member A is a protein that in humans is encoded by the CLEC4A gene.

Xerocomus subtomentosus, commonly known as suede bolete, brown and yellow bolete , boring brown bolete or yellow-cracked bolete, is a species of bolete fungus in the family Boletaceae. The fungus was initially described by Carl Linnaeus in 1753 and known for many years as Boletus subtomentosus. It is edible, though not as highly regarded as other bolete mushrooms.
BanLec is a lectin from the jacalin-related lectin family isolated from the fruit of the bananas Musa acuminata and Musa balbisiana. BanLec is one of the predominant proteins in the pulp of ripe bananas and has binding specificity for mannose and mannose-containing oligosaccharides. A 2010 study reported that BanLec was a potent inhibitor of HIV replication.
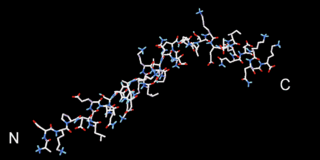
Beta thymosins are a family of proteins which have in common a sequence of about 40 amino acids similar to the small protein thymosin β4. They are found almost exclusively in multicellular animals. Thymosin β4 was originally obtained from the thymus in company with several other small proteins which although named collectively "thymosins" are now known to be structurally and genetically unrelated and present in many different animal tissues.

A circular permutation is a relationship between proteins whereby the proteins have a changed order of amino acids in their peptide sequence. The result is a protein structure with different connectivity, but overall similar three-dimensional (3D) shape. In 1979, the first pair of circularly permuted proteins – concanavalin A and lectin – were discovered; over 2000 such proteins are now known.

In molecular biology, the jacalin-like lectin domain is a mannose-binding lectin domain with a beta-prism fold consisting of three 4-stranded beta-sheets, with an internal pseudo 3-fold symmetry. Some lectins in this group stimulate distinct T- and B-cell functions, such as Jacalin, which binds to the T-antigen and acts as an agglutinin. This domain is found in 1 to 6 copies in lectins. The domain is also found in the salt-stress induced protein from rice and an animal prostatic spermine-binding protein.

Xerocomellus is a genus of fungi in the family Boletaceae. The genus, as it was described in 2008, contained 12 species. However X. rubellus and X. engelii were transferred to the new genus Hortiboletus and X. armeniacus was transferred to the new genus Rheubarbariboletus in 2015. Molecular analysis supports the distinction of Xerocomellus species from Boletus and Xerocomus, within which these species were formerly contained. Xerocomellus in fact is only distantly related to Xerocomus and is most closely related to Tylopilus, Boletus sensu stricto, Porphyrellus, Strobilomyces, and Xanthoconium.
A protein superfamily is the largest grouping (clade) of proteins for which common ancestry can be inferred. Usually this common ancestry is inferred from structural alignment and mechanistic similarity, even if no sequence similarity is evident. Sequence homology can then be deduced even if not apparent. Superfamilies typically contain several protein families which show sequence similarity within each family. The term protein clan is commonly used for protease and glycosyl hydrolases superfamilies based on the MEROPS and CAZy classification systems.

Intelectins are lectins expressed in humans and other chordates. Humans express two types of intelectins encoded by ITLN1 and ITLN2 genes respectively. Several intelectins bind microbe-specific carbohydrate residues. Therefore, intelectins have been proposed to function as immune lectins. Even though intelectins contain fibrinogen-like domain found in the ficolins family of immune lectins, there is significant structural divergence. Thus, intelectins may not function through the same lectin-complement pathway. Most intelectins are still poorly characterized and they may have diverse biological roles. Human intelectin-1 (hIntL-1) has also been shown to bind lactoferrin, but the functional consequence has yet to be elucidated. Additionally, hIntL-1 is a major component of asthmatic mucus and may be involved in insulin physiology as well.

Cry6Aa is a toxic crystal protein generated by the bacterial family Bacillus thuringiensis during sporulation. This protein is a member of the alpha pore forming toxins family, which gives it insecticidal qualities advantageous in agricultural pest control. Each Cry protein has some level of target specificity; Cry6Aa has specific toxic action against coleopteran insects and nematodes. The corresponding B. thuringiensis gene, cry6aa, is located on bacterial plasmids. Along with several other Cry protein genes, cry6aa can be genetically recombined in Bt corn and Bt cotton so the plants produce specific toxins. Insects are developing resistance to the most commonly inserted proteins like Cry1Ac. Since Cry6Aa proteins function differently than other Cry proteins, they are combined with other proteins to decrease the development of pest resistance. Recent studies suggest this protein functions better in combination with other virulence factors such as other Cry proteins and metalloproteinases.>
Dactylellina haptotyla is a common soil-living fungus that develops structures to capture nematodes as nutrient source. In the presence of nematodes, spores can germinate into sticky knobs or non-constricting loops. The fungus traps nematodes with sticky knobs and non-constricting loops, then breakdown the cuticle, and penetrates the body of nematodes to obtain nutrients. For its predatory nature, Dactylellina haptotyla is also considered as nematode-trapping fungus or carnivorous fungus.
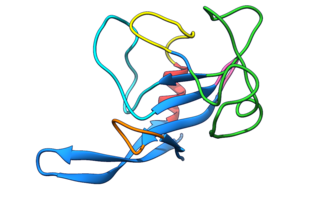
Fungal ribotoxins are a group of extracellular ribonucleases (RNases) secreted by fungi. Their most notable characteristic is their extraordinary specificity. They inactivate ribosomes by cutting a single phosphodiester bond of the rRNA that is found in a universally conserved sequence. This cleavage leads to cell death by apoptosis. However, since they are extracellular proteins, they must first enter the cells that constitute their target to exert their cytotoxic action. This entry constitutes the rate-determining step of their action.















