A cisterna are all of the membrane-bound sacs that could be found in both the Golgi apparatus and in the Endoplasmic Reticulum. Cisterna are an integral part of the packaging and modification processes of proteins occurring in the Golgi. It is the flattened sac on the branch of the Endoplasmic Reticulum and the curved sac on the branch of the Golgi apparatus. The proteins begin on the cis side of the Golgi and exit on the trans side. Throughout their journey in the cisterna, the proteins are packaged and are modified for transport throughout the cell. The number of cisterna in the Golgi stack is dependent on the organism and cell type. The structure, composition, and function of each of the cisternae may be different inside the Golgi stack. These different variations of Golgi cisternae are categorized into 3 groups; cis golgi network, medial, and trans Golgi network. The cis Golgi network is the first step in the cisternal structure of a protein being packaged, while the trans Golgi network is the last step in the cisternal structure when the vesicle is being transferred to either the lysosome, the cell surface or the secretory vesicle. The cisternae are shaped by the cytoskeleton of the cell through a lipid bilayer. Post-translational modifications such as glycosylation, phosphorylation and cleavage occur in the Golgi and as proteins travel through it, they go through the cisternae, which allows functional ion channels to be created due to these modifications. Each class of cisternae contains various enzymes used in protein modifications. These enzymes help the Golgi in glycosylation and phosphorylation of proteins, as well as mediate signal modifications to direct proteins to their final destination. Defects in the cisternal enzymes can cause congenital defects including some forms of muscular dystrophy, cystic fibrosis, cancer, and diabetes.

Dynactin subunit 1 is a protein that in humans is encoded by the DCTN1 gene.

Golgin subfamily A member 2 is a protein that in humans is encoded by the GOLGA2 gene.

General vesicular transport factor p115 is a protein that in humans is encoded by the USO1 gene.

Dynactin subunit 2 is a protein that in humans is encoded by the DCTN2 gene

Ras-related protein Rab-1A is a protein that in humans is encoded by the RAB1A gene.

Golgi reassembly-stacking protein of 65 kDa (GRASP65) also known as Golgi reassembly-stacking protein 1 (GORASP1) is a protein that in humans is encoded by the GORASP1 gene.

Syntaxin-5 is a protein that in humans is encoded by the STX5 gene.

Kinesin-like protein KIF20A is a protein that in humans is encoded by the KIF20A gene.

Transmembrane emp24 domain-containing protein 10 is a protein that in humans is encoded by the TMED10 gene.

Golgin subfamily A member 3 is a protein that in humans is encoded by the GOLGA3 gene.
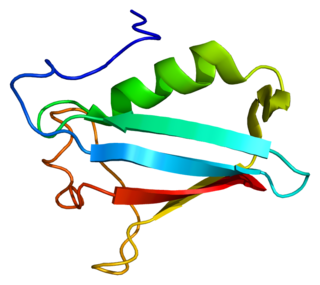
NSFL1 cofactor p47 is a protein that in humans is encoded by the NSFL1C gene.
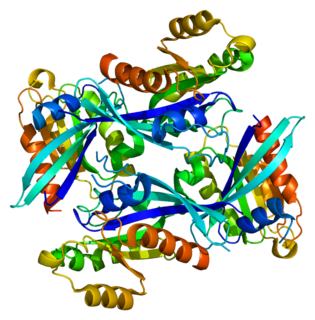
Ras-related protein Rab-2A is a protein that in humans is encoded by the RAB2A gene.

Bicaudal D cargo adaptor 1 is a protein that in humans is encoded by the BICD1 gene.

Golgin subfamily A member 5 is a protein that in humans is encoded by the GOLGA5 gene.

Golgin-45 is a protein that in humans is encoded by the BLZF1 gene.

Kinesin-like protein KIFC1 is a protein that in humans is encoded by the KIFC1 gene.

Transmembrane emp24 domain-containing protein 2 is a protein that in humans is encoded by the TMED2 gene.

The Golgi matrix is a collection of proteins involved in the structure and function of the Golgi apparatus. The matrix was first isolated in 1994 as an amorphous collection of 12 proteins that remained associated together in the presence of detergent and 150 mM NaCl. Treatment with a protease enzyme removed the matrix, which confirmed the importance of proteins for the matrix structure. Modern freeze etch electron microscopy (EM) clearly shows a mesh connecting Golgi cisternae and associated vesicles. Further support for the existence of a matrix comes from EM images showing that ribosomes are excluded from regions between and near Golgi cisternae.
Olwyn Byron is a British physicist who is Professor of Biophysics at the University of Glasgow and Chair of the British Biophysical Society. She was a member of the Physics of Life UK Network steering group who were awarded the 2020 Institute of Physics Rosalind Franklin Medal and Prize.






















