Polycystic ovary syndrome, or polycystic ovarian syndrome (PCOS), is the most common endocrine disorder in women of reproductive age. The syndrome is named after cysts which form on the ovaries of some people with this condition, though this is not a universal symptom, and not the underlying cause of the disorder.

Type 2 diabetes (T2D), formerly known as adult-onset diabetes, is a form of diabetes mellitus that is characterized by high blood sugar, insulin resistance, and relative lack of insulin. Common symptoms include increased thirst, frequent urination, fatigue and unexplained weight loss. Symptoms may also include increased hunger, having a sensation of pins and needles, and sores (wounds) that do not heal. Often symptoms come on slowly. Long-term complications from high blood sugar include heart disease, strokes, diabetic retinopathy which can result in blindness, kidney failure, and poor blood flow in the limbs which may lead to amputations. The sudden onset of hyperosmolar hyperglycemic state may occur; however, ketoacidosis is uncommon.
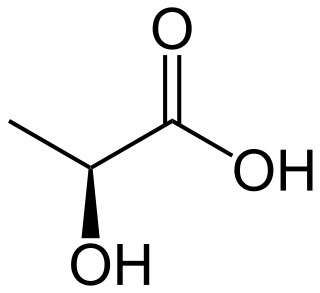
Lactic acidosis is a medical condition characterized by a build-up of lactate in the body, with formation of an excessively low pH in the bloodstream. It is a form of metabolic acidosis, in which excessive acid accumulates due to a problem with the body's oxidative metabolism.
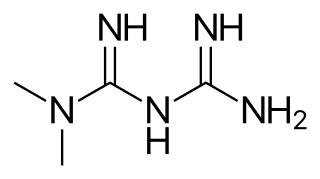
Metformin, sold under the brand name Glucophage, among others, is the main first-line medication for the treatment of type 2 diabetes, particularly in people who are overweight. It is also used in the treatment of polycystic ovary syndrome. It is sometimes used as an off-label adjunct to lessen the risk of metabolic syndrome in people who take antipsychotics. Metformin is not associated with weight gain and is taken by mouth.
Drugs used in diabetes treat diabetes mellitus by decreasing glucose levels in the blood. With the exception of insulin, most GLP-1 receptor agonists, and pramlintide, all diabetes medications are administered orally and are thus called oral hypoglycemic agents or oral antihyperglycemic agents. There are different classes of hypoglycemic drugs, and selection of the appropriate agent depends on the nature of diabetes, age, and situation of the person, as well as other patient factors.

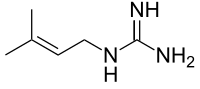
Galega officinalis, commonly known as galega or goat's-rue, is a herbaceous plant in the subfamily Faboideae of the legume family Fabaceae. It is native to parts of northern Africa, western Asia and Europe, but is widely cultivated and naturalised elsewhere. The plant has been extensively cultivated as a forage crop, an ornamental, a bee plant, and as green manure.

Pioglitazone, sold under the brand name Actos among others, is an anti-diabetic medication used to treat type 2 diabetes. It may be used with metformin, a sulfonylurea, or insulin. Use is recommended together with exercise and diet. It is not recommended in type 1 diabetes. It is taken by mouth.
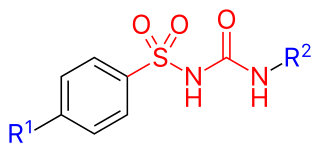
Sulfonylureas or sulphonylureas are a class of organic compounds used in medicine and agriculture. The functional group consists of a sulfonyl group (-S(=O)2) with its sulphur atom bonded to a nitrogen atom of a ureylene group (N,N-dehydrourea, a dehydrogenated derivative of urea). The side chains R1 and R2 distinguish various sulfonylureas. Sulfonylureas are the most widely used herbicide.

Biguanide is the organic compound with the formula HN(C(NH)NH2)2. It is a colorless solid that dissolves in water to give highly basic solution. These solutions slowly hydrolyse to ammonia and urea.

Vildagliptin, sold under the brand name Galvus and others, is an oral anti-hyperglycemic agent of the dipeptidyl peptidase-4 (DPP-4) inhibitor class of drugs. Vildagliptin inhibits the inactivation of GLP-1 and GIP by DPP-4, allowing GLP-1 and GIP to potentiate the secretion of insulin in the beta cells and suppress glucagon release by the alpha cells of the islets of Langerhans in the pancreas.

Inhibitors of dipeptidyl peptidase 4 are a class of oral hypoglycemics that block the enzyme dipeptidyl peptidase-4 (DPP-4). They can be used to treat diabetes mellitus type 2.

Sitagliptin, sold under the brand name Januvia among others, is an anti-diabetic medication used to treat type 2 diabetes. In the United Kingdom it is listed as less preferred than metformin or a sulfonylurea. It is taken by mouth. It is also available in the fixed-dose combination medication sitagliptin/metformin.

Saxagliptin, sold under the brand name Onglyza, is an oral hypoglycemic of the dipeptidyl peptidase-4 (DPP-4) inhibitor class. Early development was solely by Bristol-Myers Squibb; in 2007 AstraZeneca joined with Bristol-Myers Squibb to co-develop the final compound and collaborate on the marketing of the drug.
Dapagliflozin, sold under the brand names Farxiga (US) and Forxiga (EU) among others, is a medication used to treat type 2 diabetes. It is also used to treat adults with heart failure and chronic kidney disease. It reversibly inhibits sodium-glucose co-transporter 2 (SGLT-2) in the renal proximal convoluted tubule to reduce glucose reabsorption and increase urinary glucose excretion.

N-Nitrosodimethylamine (NDMA), also known as dimethylnitrosamine (DMN), is an organic compound with the formula (CH3)2NNO. It is one of the simplest members of a large class of nitrosamines. It is a volatile yellow oil. NDMA has attracted wide attention as being highly hepatotoxic and a known carcinogen in laboratory animals.

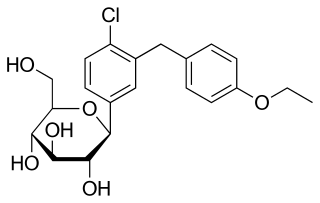
Remogliflozin etabonate (INN/USAN) is a drug of the gliflozin class for the treatment of non-alcoholic steatohepatitis ("NASH") and type 2 diabetes. Remogliflozin was discovered by the Japanese company Kissei Pharmaceutical and is currently being developed by BHV Pharma, a wholly owned subsidiary of North Carolina, US-based Avolynt, and Glenmark Pharmaceuticals through a collaboration with BHV. In 2002, GlaxoSmithKline (GSK) received a license to use it. From 2002 to 2009, GSK carried out a significant clinical development program for the treatment of type-2 diabetes mellitus in various nations across the world and obesity in the UK. Remogliflozin etabonate's pharmacokinetics, pharmacodynamics, and clinical dose regimens were characterized in 18 Phase I and 2 Phase II investigations. Due to financial concerns, GSK stopped working on remogliflozin and sergliflozin, two further SGLT2 inhibitors that were licensed to the company, in 2009. Remogliflozin was commercially launched first in India by Glenmark in May 2019.
Synthalin was an oral anti-diabetic drug. Discovered in 1926 it was marketed in Europe by Schering AG of Berlin as a synthetic drug with insulin-like properties that could be taken orally. However, it was toxic to the liver and kidney and was withdrawn from the market in the early 1940s.

Trelagliptin is a pharmaceutical drug used for the treatment of type 2 diabetes.

Gosogliptin is a drug for the treatment of type II diabetes. It is in the class of dipeptidyl peptidase-4 (DPP-4) inhibitors. It was discovered and developed through Phase 1 and Phase 2 by Pfizer. The crystal structure of DPP-4 in complex with gosogliptin is available. Its metabolism, excretion and pharmacokinetics in rat, dog and human have been described. A cost efficient route has been published. Other studies including Phase 3 studies were conducted in Russia. It is approved for use in Russia.

Ertugliflozin, sold under the brand name Steglatro, is a medication for the treatment of type 2 diabetes.