Related Research Articles

In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation. Chaperones are also involved in the translocation of proteins for proteolysis.

Ribulose-1,5-bisphosphate carboxylase/oxygenase, commonly known by the abbreviations RuBisCo, rubisco, RuBPCase, or RuBPco, is an enzyme involved in light-independent part of photosynthesis, including the carbon fixation by which atmospheric carbon dioxide is converted by plants and other photosynthetic organisms to energy-rich molecules such as glucose. It emerged approximately four billion years ago in primordial metabolism prior to the presence of oxygen on earth. It is probably the most abundant enzyme on Earth. In chemical terms, it catalyzes the carboxylation of ribulose-1,5-bisphosphate.
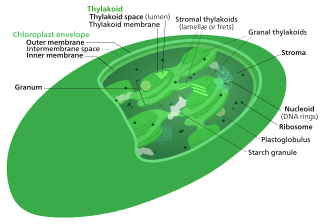
The Calvin cycle,light-independent reactions, bio synthetic phase,dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into glucose. The Calvin cycle is present in all photosynthetic eukaryotes and also many photosynthetic bacteria. In plants, these reactions occur in the stroma, the fluid-filled region of a chloroplast outside the thylakoid membranes. These reactions take the products of light-dependent reactions and perform further chemical processes on them. The Calvin cycle uses the chemical energy of ATP and reducing power of NADPH from the light dependent reactions to produce sugars for the plant to use. These substrates are used in a series of reduction-oxidation reactions to produce sugars in a step-wise process; there is no direct reaction that converts several molecules of CO2 to a sugar. There are three phases to the light-independent reactions, collectively called the Calvin cycle: carboxylation, reduction reactions, and ribulose 1,5-bisphosphate (RuBP) regeneration.

Sir Alan Roy Fersht is a British chemist at the MRC Laboratory of Molecular Biology, Cambridge, and an Emeritus Professor in the Department of Chemistry at the University of Cambridge. He was Master of Gonville and Caius College, Cambridge from 2012 to 2018. He works on protein folding, and is sometimes described as a founder of protein engineering.

GroEL is a protein which belongs to the chaperonin family of molecular chaperones, and is found in many bacteria. It is required for the proper folding of many proteins. To function properly, GroEL requires the lid-like cochaperonin protein complex GroES. In eukaryotes the organellar proteins Hsp60 and Hsp10 are structurally and functionally nearly identical to GroEL and GroES, respectively, due to their endosymbiotic origin.
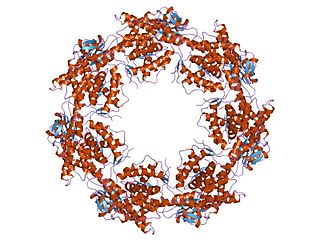
HSP60, also known as chaperonins (Cpn), is a family of heat shock proteins originally sorted by their 60kDa molecular mass. They prevent misfolding of proteins during stressful situations such as high heat, by assisting protein folding. HSP60 belong to a large class of molecules that assist protein folding, called molecular chaperones.

Sir John Ernest Walker is a British chemist who won the Nobel Prize in Chemistry in 1997. As of 2015 Walker is Emeritus Director and Professor at the MRC Mitochondrial Biology Unit in Cambridge, and a Fellow of Sidney Sussex College, Cambridge.

Propionyl-CoA carboxylase (EC 6.4.1.3, PCC) catalyses the carboxylation reaction of propionyl-CoA in the mitochondrial matrix. PCC has been classified both as a ligase and a lyase. The enzyme is biotin-dependent. The product of the reaction is (S)-methylmalonyl CoA.
Non-chaperonin molecular chaperone ATPase (EC 3.6.4.10, molecular chaperone Hsc70 ATPase) is an enzyme with systematic name ATP phosphohydrolase (polypeptide-polymerizing). This enzyme catalyses the following chemical reaction

Malate dehydrogenase (oxaloacetate-decarboxylating) (NADP+) (EC 1.1.1.40) or NADP-malic enzyme (NADP-ME) is an enzyme that catalyzes the chemical reaction in the presence of a bivalent metal ion:
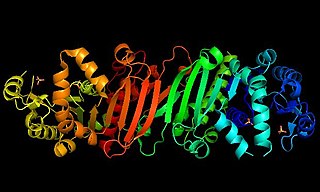
Phosphoribulokinase (PRK) (EC 2.7.1.19) is an essential photosynthetic enzyme that catalyzes the ATP-dependent phosphorylation of ribulose 5-phosphate (RuP) into ribulose 1,5-bisphosphate (RuBP), both intermediates in the Calvin Cycle. Its main function is to regenerate RuBP, which is the initial substrate and CO2-acceptor molecule of the Calvin Cycle. PRK belongs to the family of transferase enzymes, specifically those transferring phosphorus-containing groups (phosphotransferases) to an alcohol group acceptor. Along with ribulose 1,5-bisphosphate carboxylase/oxygenase (RuBisCo), phosphoribulokinase is unique to the Calvin Cycle. Therefore, PRK activity often determines the metabolic rate in organisms for which carbon fixation is key to survival. Much initial work on PRK was done with spinach leaf extracts in the 1950s; subsequent studies of PRK in other photosynthetic prokaryotic and eukaryotic organisms have followed. The possibility that PRK might exist was first recognized by Weissbach et al. in 1954; for example, the group noted that carbon dioxide fixation in crude spinach extracts was enhanced by the addition of ATP. The first purification of PRK was conducted by Hurwitz and colleagues in 1956.
ATP + Mg2+ - D-ribulose 5-phosphate ADP + D-ribulose 1,5-bisphosphate

Dame Carol Vivien Robinson, is a British chemist and former president of the Royal Society of Chemistry (2018–2020). She was a Royal Society Research Professor and is the Dr Lee's Professor of Physical and Theoretical Chemistry, and a professorial fellow at Exeter College, University of Oxford. She is the first director of the Kavli Institution for Nanoscience Discovery, University of Oxford, and she was previously professor of mass spectrometry at the chemistry department of the University of Cambridge.

Sir Christopher Martin Dobson was a British chemist, who was the John Humphrey Plummer Professor of Chemical and Structural Biology in the Department of Chemistry at the University of Cambridge, and Master of St John's College, Cambridge.
Fraser Andrew Armstrong is a professor of chemistry at the University of Oxford and a Fellow of St John's College, Oxford.
Johannes Buchner is a German biochemist and professor at the Technische Universität München, Munich, Germany.
Sheena Elizabeth Radford FRS FMedSci is a British biophysicist, and Astbury Professor of Biophysics in the Astbury Centre for Structural Molecular Biology, School of Molecular and Cellular Biology at the University of Leeds. Radford is the Associate Editor of the Journal of Molecular Biology.

Annette Catherine Dolphin is a Professor of Pharmacology in the Department of Neuroscience, Physiology and Pharmacology at University College London (UCL).

The kinetic isotope effect (KIE) of ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCO) is the isotopic fractionation associated solely with the step in the Calvin-Benson cycle where a molecule of carbon dioxide is attached to the 5-carbon sugar ribulose-1,5-bisphosphate (RuBP) to produce two 3-carbon sugars called 3-phosphoglycerate. This chemical reaction is catalyzed by the enzyme RuBisCO, and this enzyme-catalyzed reaction creates the primary kinetic isotope effect of photosynthesis. It is also largely responsible for the isotopic compositions of photosynthetic organisms and the heterotrophs that eat them. Understanding the intrinsic KIE of RuBisCO is of interest to earth scientists, botanists, and ecologists because this isotopic biosignature can be used to reconstruct the evolution of photosynthesis and the rise of oxygen in the geologic record, reconstruct past evolutionary relationships and environmental conditions, and infer plant relationships and productivity in modern environments.

Pierre A. Goloubinoff is a Swiss-Israeli biochemist, author and activist for Yemeni immigration and the preservation of Yemeni heritage. He is also known by the pen names David Hamami or Daoud Hamami.

T-complex protein Ring Complex (TRiC), otherwise known as Chaperonin Containing TCP-1 (CCT), is a multiprotein complex and the chaperonin of eukaryotic cells. Like the bacterial GroEL, the TRiC complex aids in the folding of ~10% of the proteome, and actin and tubulin are some of its best known substrates. TRiC is an example of a biological machine that folds substrates within the central cavity of its barrel-like assembly using the energy from ATP hydrolysis.
References
- 1 2 3 4 Anon (2017). "Lorimer, Prof. George Huntly" . Who's Who (online Oxford University Press ed.). Oxford: A & C Black. doi:10.1093/ww/9780199540884.013.24943.(Subscription or UK public library membership required.)
- 1 2 3 4 5 6 7 8 9 Anon (1986). "Professor George Lorimer FRS". royalsociety.org. Royal Society. Archived from the original on 2016-04-10. One or more of the preceding sentences incorporates text from the royalsociety.org website where:
“All text published under the heading 'Biography' on Fellow profile pages is available under Creative Commons Attribution 4.0 International License.” -- "Royal Society Terms, conditions and policies". Archived from the original on 2016-11-11. Retrieved 2016-03-09.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - 1 2 "Department of Chemistry and Biochemistry – University of Maryland, College Park MD George Lorimer - Department of Chemistry and Biochemistry". www.chem.umd.edu. Archived from the original on 2017-09-28.
![]() This article incorporates text available under the CC BY 4.0 license.
This article incorporates text available under the CC BY 4.0 license.