
Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene is a major component of the common solvent, turpentine.

An essential oil is a concentrated hydrophobic liquid containing volatile chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the oil of the plant from which they were extracted, such as oil of clove. An essential oil is essential in the sense that it contains the essence of the plant's fragrance—the characteristic fragrance of the plant from which it is derived. The term "essential" used here does not mean indispensable or usable by the human body, as with the terms essential amino acid or essential fatty acid, which are so called because they are nutritionally required by a living organism.

Used in perfumery and aromatherapy, absolutes are similar to essential oils. They are concentrated, highly aromatic, oily mixtures extracted from plants. Whereas essential oils are produced by distillation, boiling or pressing, absolutes are produced through solvent extraction, or more traditionally, through enfleurage.

Citronella oil is an essential oil obtained from the leaves and stems of different species of Cymbopogon (lemongrass). The oil is used extensively as a source of perfumery chemicals such as citronellal, citronellol, and geraniol. These chemicals find extensive use in soap, candles and incense, perfumery, cosmetic, and flavouring industries throughout the world. Citronella oil is also a plant-based insect repellent and has been registered for this use in the United States since 1948. The United States Environmental Protection Agency considers oil of citronella as a biopesticide with a non-toxic mode of action.

Geraniol is a monoterpenoid and an alcohol. It is the primary component of citronella oil and is a primary component of rose oil, palmarosa oil. It is a colorless oil, although commercial samples can appear yellow. It has low solubility in water, but it is soluble in common organic solvents. The functional group derived from geraniol is called geranyl.

Linalool refers to two enantiomers of a naturally occurring terpene alcohol found in many flowers and spice plants. Linalool has multiple commercial applications, the majority of which are based on its pleasant scent. A colorless oil, linalool is classified as an acyclic monoterpenoid. In plants, it is a metabolite, a volatile oil component, an antimicrobial agent, and an aroma compound. Linalool has uses in manufacturing of soaps, fragrances, food additives as flavors, household products, and insecticides. Esters of linalool are referred to as linalyl, e.g. linalyl pyrophosphate, an isomer of geranyl pyrophosphate.

Rose oil is the essential oil extracted from the petals of various types of rose. Rose ottos are extracted through steam distillation, while rose absolutes are obtained through solvent extraction, the absolute being used more commonly in perfumery. The production technique originated in Greater Iran. Even with their high price and the advent of organic synthesis, rose oils are still perhaps the most widely used essential oil in perfumery.
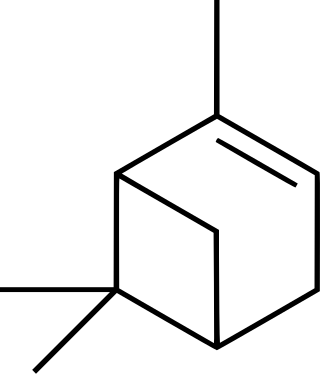
Pinene is a collection of unsaturated bicyclic monoterpenes. Two geometric isomers of pinene are found in nature, α-pinene and β-pinene. Both are chiral. As the name suggests, pinenes are found in pines. Specifically, pinene is the major component of the liquid extracts of conifers. Pinenes are also found in many non-coniferous plants such as camphorweed (Heterotheca) and big sagebrush.

Myrcene, or β-myrcene, is a monoterpene. A colorless oil, it occurs widely in essential oils. It is produced mainly semi-synthetically from Myrcia, from which it gets its name. It is an intermediate in the production of several fragrances. α-Myrcene is the name for the isomer 2-methyl-6-methylene-1,7-octadiene, which has not been found in nature.

Caryophyllene, more formally (−)-β-caryophyllene,(BCP), is a natural bicyclic sesquiterpene that is a constituent of many essential oils, especially clove oil, the oil from the stems and flowers of Syzygium aromaticum (cloves), the essential oil of Cannabis sativa, copaiba, rosemary, and hops. It is usually found as a mixture with isocaryophyllene (the cis double bond isomer) and α-humulene (obsolete name: α-caryophyllene), a ring-opened isomer. Caryophyllene is notable for having a cyclobutane ring, as well as a trans-double bond in a 9-membered ring, both rarities in nature.

Citronellol, or dihydrogeraniol, is a natural acyclic monoterpenoid. Both enantiomers occur in nature. (+)-Citronellol, which is found in citronella oils, including Cymbopogon nardus (50%), is the more common isomer. (−)-Citronellol is widespread, but particularly abundant in the oils of rose (18–55%) and Pelargonium geraniums.
Camphene is a bicyclic organic compound. It is one of the most pervasive monoterpenes. As with other terpenes, it is insoluble in water, flammable, colorless, and has a pungent smell. It is a minor constituent of many essential oils such as turpentine, cypress oil, camphor oil, citronella oil, neroli, ginger oil, valerian, and mango. It is produced industrially by isomerization of the more common alpha-pinene using a solid acid catalyst such as titanium dioxide.

Nerolidol, also known as peruviol and penetrol, is a naturally occurring sesquiterpene alcohol. A colorless liquid, it is found in the essential oils of many types of plants and flowers. There are four isomers of nerolidol', which differ in the geometry about the central double bond and configuration of the hydroxyl-bearing carbon, but most applications use such a mixture. The aroma of nerolidol is woody and reminiscent of fresh bark. It is used as a flavoring agent and in perfumery as well as in non-cosmetic products such as detergents and cleansers. Nerolidyl derivatives include nerolidyl diphosphate and the fragrance nerolidyl acetate.
The terpinenes are a group of isomeric hydrocarbons that are classified as monoterpenes. They each have the same molecular formula and carbon framework, but they differ in the position of carbon-carbon double bonds. α-Terpinene has been isolated from cardamom and marjoram oils, and from other natural sources. β-Terpinene has no known natural source but has been prepared from sabinene. γ-Terpinene and δ-terpinene have been isolated from a variety of plant sources. They are all colorless liquids with a turpentine-like odor.

Eucalyptus oil is the generic name for distilled oil from the leaf of Eucalyptus, a genus of the plant family Myrtaceae native to Australia and cultivated worldwide. Eucalyptus oil has a history of wide application, as a pharmaceutical, antiseptic, repellent, flavouring, fragrance and industrial uses. The leaves of selected Eucalyptus species are steam distilled to extract eucalyptus oil.

Cannabis flower essential oil, also known as hemp essential oil, is an essential oil obtained by steam distillation from the flowers, panicles, stem, and upper leaves of the hemp plant. Hemp essential oil is distinct from hemp seed oil and hash oil: the former is a vegetable oil that is cold-pressed from the seeds of low-THC varieties of hemp, the latter is a THC-rich extract of dried female hemp flowers (marijuana) or resin (hashish).

Methyl anthranilate, also known as MA, methyl 2-aminobenzoate, or carbomethoxyaniline, is an ester of anthranilic acid. Its chemical formula is C8H9NO2. It has a strong and fruity grape smell, and one of its key uses is as a flavoring agent.

Linalyl acetate is an organic compound, the acetate ester of linalool and a phytochemical found in many flowers and spice plants. It is one of the principal components of the essential oils of bergamot and lavender. It often occurs together with linalool and is a widely used fragrance.
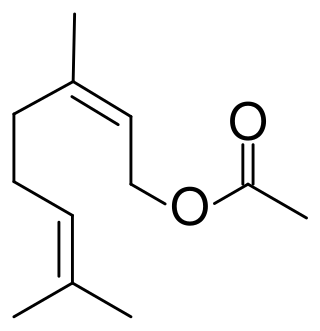
Neryl acetate is a terpenoid found in citrus oils. It is the acetate ester of nerol, an isomer of the more common fragrance geranyl acetate. In flavors and perfumery it is used to impart floral and fruity aromas.
Geranylacetone is an organic compound with the formula CH3C(O)(CH2)2CH=C(CH3)(CH2)2CH=C(CH3)2. A colorless oil, it is the product of coupling geranyl and acetonyl groups. It is a precursor to synthetic squalene.


















