Threonine is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, a carboxyl group, and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as E. coli. It is encoded by all the codons starting AC.
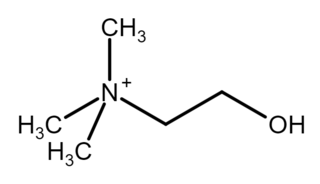
Choline is a cation with the chemical formula [(CH3)3NCH2CH2OH]+. Choline forms various salts, for example choline chloride and choline bitartrate.
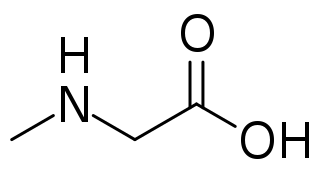
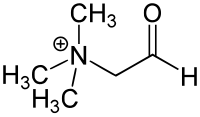
Sarcosine, also known as N-methylglycine, or monomethylglycine, is a amino acid with the formula CH3N(H)CH2CO2H. It exists at neutral pH as the zwitterion CH3N+(H)2CH2CO2−, which can be obtained as a white, water-soluble powder. Like some amino acids, sarcosine converts to a cation at low pH and an anion at high pH, with the respective formulas CH3N+(H)2CH2CO2H and CH3N(H)CH2CO2−. Sarcosine is a close relative of glycine, with a secondary amine in place of the primary amine.
Lipotropic compounds are those that help catalyse the breakdown of fat during metabolism in the body. A lipotropic nutrient promotes or encourages the export of fat from the liver. Lipotropics are necessary for maintenance of a healthy liver, and for burning the exported fat for additional energy. Without lipotropics, such as choline and inositol, fats and bile can become trapped in the liver, causing severe problems such as cirrhosis and blocking fat metabolism.

Trimethylglycine is an amino acid derivative that occurs in plants. Trimethylglycine was the first betaine discovered; originally it was simply called betaine because, in the 19th century, it was discovered in sugar beets.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).

Serine hydroxymethyltransferase (SHMT) is a pyridoxal phosphate (PLP) (Vitamin B6) dependent enzyme (EC 2.1.2.1) which plays an important role in cellular one-carbon pathways by catalyzing the reversible, simultaneous conversions of L-serine to glycine and tetrahydrofolate (THF) to 5,10-Methylenetetrahydrofolate (5,10-CH2-THF). This reaction provides the largest part of the one-carbon units available to the cell.
In enzymology, a L-threonine 3-dehydrogenase (EC 1.1.1.103) is an enzyme that catalyzes the chemical reaction
In enzymology, a (R)-aminopropanol dehydrogenase (EC 1.1.1.75) is an enzyme that catalyzes the chemical reaction
In the field of enzymology, a betaine-homocysteine S-methyltransferase also known as betaine-homocysteine methyltransferase (BHMT) is a zinc metallo-enzyme that catalyzes the transfer of a methyl group from trimethylglycine and a hydrogen ion from homocysteine to produce dimethylglycine and methionine respectively:
In enzymology, a choline monooxygenase (EC 1.14.15.7) is an enzyme that catalyzes the chemical reaction
In enzymology, a choline dehydrogenase is an enzyme that catalyzes the chemical reaction
In enzymology, a choline oxidase (EC 1.1.3.17) is an enzyme that catalyzes the chemical reaction

In enzymology, an aspartate-semialdehyde dehydrogenase is an enzyme that is very important in the biosynthesis of amino acids in prokaryotes, fungi, and some higher plants. It forms an early branch point in the metabolic pathway forming lysine, methionine, leucine and isoleucine from aspartate. This pathway also produces diaminopimelate which plays an essential role in bacterial cell wall formation. There is particular interest in ASADH as disabling this enzyme proves fatal to the organism giving rise to the possibility of a new class of antibiotics, fungicides, and herbicides aimed at inhibiting it.

In enzymology, a betaine-aldehyde dehydrogenase (EC 1.2.1.8) is an enzyme that catalyzes the chemical reaction
In enzymology, a dimethylglycine dehydrogenase (EC 1.5.8.4) is an enzyme that catalyzes the chemical reaction
In enzymology, a glycine dehydrogenase (cytochrome) (EC 1.4.2.1) is an enzyme that catalyzes the chemical reaction

The enzyme threonine aldolase is an enzyme that catalyzes the chemical reaction

Glycocyamine is a metabolite of glycine in which the amino group has been converted into a guanidine by guanylation. In vertebrate organism it is then transformed into creatine by methylation.
Carnitine biosynthesis is a method for the endogenous production of L-carnitine, a molecule that is essential for energy metabolism. In humans and many other animals, L-carnitine is obtained from both diet and by biosynthesis. The carnitine biosynthesis pathway is highly conserved among many eukaryotes and some prokaryotes.