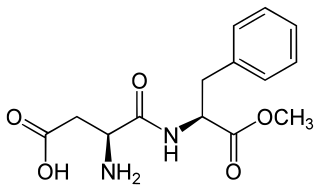
Aspartame is an artificial non-saccharide sweetener 200 times sweeter than sucrose and is commonly used as a sugar substitute in foods and beverages. It is a methyl ester of the aspartic acid/phenylalanine dipeptide with brand names NutraSweet, Equal, and Canderel. Aspartame was approved by the US Food and Drug Administration (FDA) in 1974, and then again in 1981, after approval was revoked in 1980.

Stevia is a sweet sugar substitute that is about 50 to 300 times sweeter than sugar. It is extracted from the leaves of Stevia rebaudiana, a plant native to areas of Paraguay and Brazil in the southern Amazon rainforest. The active compounds in stevia are steviol glycosides. Stevia is heat-stable, pH-stable, and not fermentable. Humans cannot metabolize the glycosides in stevia, and therefore it has zero calories. Its taste has a slower onset and longer duration than that of sugar, and at high concentrations some of its extracts may have an aftertaste described as licorice-like or bitter. Stevia is used in sugar- and calorie-reduced food and beverage products as an alternative for variants with sugar.

Fructose, or fruit sugar, is a ketonic simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed by the gut directly into the blood of the portal vein during digestion. The liver then converts both fructose and galactose into glucose, so that dissolved glucose, known as blood sugar, is the only monosaccharide present in circulating blood.

A sugar substitute is a food additive that provides a sweetness like that of sugar while containing significantly less food energy than sugar-based sweeteners, making it a zero-calorie or low-calorie sweetener. Artificial sweeteners may be derived through manufacturing of plant extracts or processed by chemical synthesis. Sugar substitute products are commercially available in various forms, such as small pills, powders, and packets.

Saccharin, also called saccharine, benzosulfimide, or E954, or used in saccharin sodium or saccharin calcium forms, is a non-nutritive artificial sweetener. Saccharin is a sultam that is about 500 times sweeter than sucrose, but has a bitter or metallic aftertaste, especially at high concentrations. It is used to sweeten products, such as drinks, candies, baked goods, tobacco products, excipients, and for masking the bitter taste of some medicines. It appears as white crystals and is odorless.

In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of Heliconius butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body.
Saponins, also selectively referred to as triterpene glycosides, are bitter-tasting usually toxic plant-derived organic chemicals that have a foamy quality when agitated in water. They are widely distributed but found particularly in soapwort, a flowering plant, the soapbark tree, common corn-cockle, baby’s breath and soybeans. They are used in soaps, medicines, fire extinguishers, as dietary supplements, for synthesis of steroids, and in carbonated beverages. Saponins are both water and fat soluble, which gives them their useful soap properties. Some examples of these chemicals are glycyrrhizin and quillaia, a bark extract used in beverages.

Lactisole is the sodium salt and commonly supplied form of 2-(4-methoxyphenoxy)propionic acid, a natural carboxylic acid found in roasted coffee beans. Like gymnemic acid, it has the property of masking sweet flavors and is used for this purpose in the food industry.

Sweetness is a basic taste most commonly perceived when eating foods rich in sugars. Sweet tastes are generally regarded as pleasurable. In addition to sugars like sucrose, many other chemical compounds are sweet, including aldehydes, ketones, and sugar alcohols. Some are sweet at very low concentrations, allowing their use as non-caloric sugar substitutes. Such non-sugar sweeteners include saccharin, aspartame, sucralose and stevia. Other compounds, such as miraculin, may alter perception of sweetness itself.

Gymnema sylvestre is a perennial woody vine native to Asia, Africa and Australia. It has been used in Ayurvedic medicine. Common names include gymnema, Australian cowplant, and Periploca of the woods, and the Hindi term gurmar, which means "sugar destroyer".

Synsepalum dulcificum is a plant in the Sapotaceae family, native to tropical Africa. It is known for its berry that, when eaten, causes sour foods subsequently consumed to taste sweet. This effect is due to miraculin. Common names for this species and its berry include miracle fruit, miracle berry, miraculous berry, sweet berry, and in West Africa, where the species originates, àgbáyun, taami, asaa, and ledidi.

Siraitia grosvenorii, also known as monk fruit, monkfruit, luohan guo, or Swingle fruit, is a herbaceous perennial vine of the gourd family, Cucurbitaceae. It is native to southern China. The plant is cultivated for its fruit extract, called mogrosides, which creates a sweetness sensation 250 times stronger than sucrose. Mogroside extract has been used as a low-calorie sweetener for drinks and in traditional Chinese medicine.

D-Psicose (C6H12O6), also known as D-allulose, or simply allulose, is a low-calorie epimer of the monosaccharide sugar fructose, used by some major commercial food and beverage manufacturers as a low-calorie sweetener. First identified in wheat in the 1940s, allulose is naturally present in small quantities in certain foods.

Steviol glycosides are the chemical compounds responsible for the sweet taste of the leaves of the South American plant Stevia rebaudiana (Asteraceae) and the main ingredients of many sweeteners marketed under the generic name stevia and several trade names. They also occur in the related species S. phlebophylla and in the plant Rubus chingii (Rosaceae).
Hodulcine are glycosides which are isolated from the leaves of Hovenia dulcis Thunb. (Rhamnaceae) also known as Japanese Raisin Tree.
Several glycosides homologue have been found in this plant and although hoduloside 1 exhibits the highest anti-sweet activity, it is less potent than gymnemic acid 1.
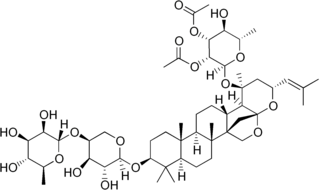
Ziziphin, a triterpene glycoside which exhibits taste-modifying properties, has been isolated from the leaves of Ziziphus jujuba (Rhamnaceae).

Pentadin, a sweet-tasting protein, was discovered and isolated in 1989, in the fruit of oubli, a climbing shrub growing in some tropical countries of Africa. Sweet tasting proteins are often used in the treatment of diabetes, obesity, and other metabolic disorders that one can experience. These proteins are isolated from the pulp of various fruits, typically found in rain forests and are also used as low calorie sweeteners that can enhance and modify existing foods.

T1R2 - Taste receptor type 1 member 2 is a protein that in humans is encoded by the TAS1R2 gene.

Gurmarin is a 35-residue polypeptide from the Asclepiad vine Gymnema sylvestre (Gurmar). It has been utilized as a pharmacological tool in the study of sweet-taste transduction because of its ability to selectively inhibit the neural response to sweet taste in rats. This rat inhibition appears to have high specificity to sugar (sweetener) molecules like sucrose, glucose, and saccharin as well as the amino acid glycine. As a sweet-taste-suppressing protein, Gurmarin shows signs of being reversible in nature although having little to no effect on the sweet taste sensation in humans suggesting the protein is only active on rodent sweet taste receptors.
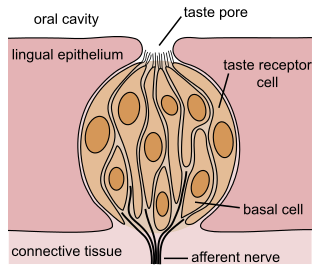
The gustatory system or sense of taste is the sensory system that is partially responsible for the perception of taste (flavor). Taste is the perception stimulated when a substance in the mouth reacts chemically with taste receptor cells located on taste buds in the oral cavity, mostly on the tongue. Taste, along with the sense of smell and trigeminal nerve stimulation, determines flavors of food and other substances. Humans have taste receptors on taste buds and other areas, including the upper surface of the tongue and the epiglottis. The gustatory cortex is responsible for the perception of taste.


















