A halohydrin dehalogenase is an enzyme involved in the bacterial degradation of vicinal halohydrins. In several species of bacteria, it catalyses the dehalogenation of halohydrins to produce the corresponding epoxides. Different isoforms of the enzyme fall into one of three groups, A, B or C. Halogenases of the same class are genetically similar, but differ greatly from halogenases from a different group. Currently the most well-studied isoform is HheC which is purified from the bacterial species Agrobacterium radiobacter. The ability to dehalogenate organic compounds as well as form enantiomeric selective epoxides have generated interest in the potential of this enzyme in the biochemical field.

A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes. An acid-base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to release the product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine or even selenocysteine. The 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).
Serine hydrolases are one of the largest known enzyme classes comprising approximately ~200 enzymes or 1% of the genes in the human proteome. A defining characteristic of these enzymes is the presence of a particular serine at the active site, which is used for the hydrolysis of substrates. The hydrolysis of the ester or peptide bond proceeds in two steps. First, the acyl part of the substrate is transferred to the serine, making a new ester or amide bond and releasing the other part of the substrate is released. Later, in a slower step, the bond between the serine and the acyl group is hydrolyzed by water or hydroxide ion, regenerating free enzyme. Unlike other, non-catalytic, serines, the reactive serine of these hydrolases is typically activated by a proton relay involving a catalytic triad consisting of the serine, an acidic residue and a basic residue, although variations on this mechanism exist.
Atrazine Chlorohydrolase (AtzA) is an enzyme (E.C.3.8.1.8), which catalyzes the conversion of atrazine to hydroxyatrazine. Bacterial degradation determines the environmental impact and efficacy of an herbicide or pesticide. Initially, most pesticides are highly effective and show minimal bacterial degradation; however, bacteria can rapidly evolve and gain the ability to metabolize potential nutrients in the environment. Despite a remarkable structural similarity, degradation of atrazine by bacteria capable of melamine degradation was rare; however, since its introduction as a pesticide in the United States, bacteria capable of atrazine degradation have evolved. Currently, Pseudomonas sp. strain ADP seems to be the optimal bacterial strain for atrazine degradations, which appears to be the sole nitrogen source for the bacteria.
In enzymology, a limonene-1,2-epoxide hydrolase (EC 3.3.2.8) is an enzyme that catalyzes the chemical reaction
In enzymology, an alkylhalidase (EC 3.8.1.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a haloacetate dehalogenase (EC 3.8.1.3) is an enzyme that catalyzes the chemical reaction
In enzymology, a (R)-2-haloacid dehalogenase(EC 3.8.1.9), DL-2-haloacid halidohydrolase (inversion of configuration), DL-DEXi, (R,S)-2-haloacid dehalogenase (configuration-inverting)) is an enzyme that catalyzes the chemical reaction
In enzymology, a (S)-2-haloacid dehalogenase (EC 3.8.1.2) is an enzyme that catalyzes the chemical reaction

In enzymology, an amidase (EC 3.5.1.4, acylamidase, acylase (misleading), amidohydrolase (ambiguous), deaminase (ambiguous), fatty acylamidase, N-acetylaminohydrolase (ambiguous)) is an enzyme that catalyzes the hydrolysis of an amide. In this way, the two substrates of this enzyme are an amide and H2O, whereas its two products are monocarboxylate and NH3.
In enzymology, an IMP cyclohydrolase (EC 3.5.4.10) is an enzyme that catalyzes the chemical reaction

Histidine kinases (HK) are multifunctional, and in non-animal kingdoms, typically transmembrane, proteins of the transferase class of enzymes that play a role in signal transduction across the cellular membrane. The vast majority of HKs are homodimers that exhibit autokinase, phosphotransfer, and phosphatase activity. HKs can act as cellular receptors for signaling molecules in a way analogous to tyrosine kinase receptors (RTK). Multifunctional receptor molecules such as HKs and RTKs typically have portions on the outside of the cell that bind to hormone- or growth factor-like molecules, portions that span the cell membrane, and portions within the cell that contain the enzymatic activity. In addition to kinase activity, the intracellular domains typically have regions that bind to a secondary effector molecule or complex of molecules that further propagate signal transduction within the cell. Distinct from other classes of protein kinases, HKs are usually parts of a two-component signal transduction mechanisms in which HK transfers a phosphate group from ATP to a histidine residue within the kinase, and then to an aspartate residue on the receiver domain of a response regulator protein. More recently, the widespread existence of protein histidine phosphorylation distinct from that of two-component histidine kinases has been recognised in human cells. In marked contrast to Ser, Thr and Tyr phosphorylation, the analysis of phosphorylated Histidine using standard biochemical and mass spectrometric approaches is much more challenging, and special procedures and separation techniques are required for their preservation alongside classical Ser, Thr and Tyr phosphorylation on proteins isolated from human cells.

Dioxygenases are oxidoreductase enzymes. Aerobic life, from simple single-celled bacteria species to complex eukaryotic organisms, has evolved to depend on the oxidizing power of dioxygen in various metabolic pathways. From energetic adenosine triphosphate (ATP) generation to xenobiotic degradation, the use of dioxygen as a biological oxidant is widespread and varied in the exact mechanism of its use. Enzymes employ many different schemes to use dioxygen, and this largely depends on the substrate and reaction at hand.

Dispersin B is a 40 kDa glycoside hydrolase produced by the periodontal pathogen, Aggregatibacter actinomycetemcomitans. The bacteria secrete Dispersin B to release adherent cells from a mature biofilm colony by disrupting biofilm formation. The enzyme catalyzes the hydrolysis of linear polymers of N-acetyl-D-glucosamines found in the biofilm matrices. Poly-acetyl glucosamines are integral to the structural integrity of the biofilms of various Gram-positive bacteria and Gram-negative bacteria and are referred to as PIA (PNAG,PS/A) in Staphylococcus species and PGA in Escherichia coli. By degrading the biofilm matrix, Dispersin B allows for the release of bacterial cells that can adhere to new surfaces close by and extend the biofilm or start new colonies. Currently there is interest in Dispersin B as a commercial anti-biofilm agent that could be combined with antibiotics for the treatment of bacterial infections.

The CFTR inhibitory factor (Cif) is a protein virulence factor secreted by the Gram-negative bacteria Pseudomonas aeruginosa and Acinetobacter nosocomialis. Discovered at Dartmouth Medical School, Cif is able to alter the trafficking of select ABC transporters in eukaryotic epithelial cells, such as the cystic fibrosis transmembrane conductance regulator (CFTR), and P-glycoprotein by interfering with the host deubiquitinating machinery. By promoting the ubiquitin-mediated degradation of CFTR, Cif is able to phenocopy cystic fibrosis at the cellular level. The cif gene is transcribed as part of a 3 gene operon, whose expression is negatively regulated by CifR, a TetR family repressor.

OmpT is an aspartyl protease found on the outer membrane of Escherichia coli. OmpT is a subtype of the family of omptin proteases, which are found on some gram-negative species of bacteria.

Neopullulanase is an enzyme of the alpha-amylase family with systematic name pullulan 4-D-glucanohydrolase (panose-forming). This enzyme principally catalyses the following chemical reaction by cleaving pullulan's alpha-1,4-glucosidic bonds:

HaloTag is a self-labeling protein tag. It is a 297 residue protein derived from a bacterial enzyme, designed to covalently bind to a synthetic ligand. The bacterial enzyme can be fused to various proteins of interest. The synthetic ligand is chosen from a number of available ligands in accordance with the type of experiments to be performed. This bacterial enzyme is a haloalkane dehalogenase, which acts as a hydrolase and is designed to facilitate visualization of the subcellular localization of a protein of interest, immobilization of a protein of interest, or capture of the binding partners of a protein of interest within its biochemical environment. The HaloTag is composed of two covalently bound segments including a haloalkane dehalogenase and a synthetic ligand of choice. These synthetic ligands consist of a reactive chloroalkane linker bound to a functional group. Functional groups can either be biotin or can be chosen from five available fluorescent dyes including Coumarin, Oregon Green, Alexa Fluor 488, diAcFAM, and TMR. These fluorescent dyes can be used in the visualization of either living or chemically fixed cells.
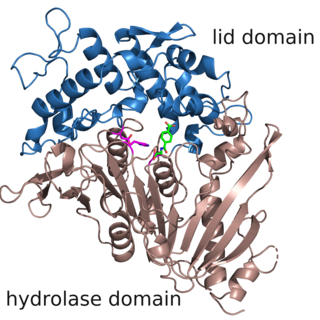
The enzyme MHETase is a hydrolase, which was discovered in 2016. It cleaves 2-hydroxyethyl terephthalic acid, the PET degradation product by PETase, to ethylene glycol and terephthalic acid. This pair of enzymes, PETase and MHETase, enable the bacterium Ideonella sakaiensis to live on the plastic PET as sole carbon source.
















