
Cytochromes are redox-active proteins containing a heme, with a central Fe atom at its core, as a cofactor. They are involved in electron transport chain and redox catalysis. They are classified according to the type of heme and its mode of binding. Four varieties are recognized by the International Union of Biochemistry and Molecular Biology (IUBMB), cytochromes a, cytochromes b, cytochromes c and cytochrome d.

Escherichia coli, also known as E. coli, is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus Escherichia that is commonly found in the lower intestine of warm-blooded organisms. Most E. coli strains are harmless, but some serotypes (EPEC, ETEC etc.) can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. Most strains do not cause disease in humans and are part of the normal microbiota of the gut; such strains are harmless or even beneficial to humans (although these strains tend to be less studied than the pathogenic ones). For example, some strains of E. coli benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by pathogenic bacteria. These mutually beneficial relationships between E. coli and humans are a type of mutualistic biological relationship — where both the humans and the E. coli are benefitting each other. E. coli is expelled into the environment within fecal matter. The bacterium grows massively in fresh faecal matter under aerobic conditions for three days, but its numbers decline slowly afterwards.

Escherichia coli O157:H7 is a serotype of the bacterial species Escherichia coli and is one of the Shiga-like toxin–producing types of E. coli. It is a cause of disease, typically foodborne illness, through consumption of contaminated and raw food, including raw milk and undercooked ground beef. Infection with this type of pathogenic bacteria may lead to hemorrhagic diarrhea, and to kidney failure; these have been reported to cause the deaths of children younger than five years of age, of elderly patients, and of patients whose immune systems are otherwise compromised.

Heme, or haem, is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.

Shiga toxins are a family of related toxins with two major groups, Stx1 and Stx2, expressed by genes considered to be part of the genome of lambdoid prophages. The toxins are named after Kiyoshi Shiga, who first described the bacterial origin of dysentery caused by Shigella dysenteriae. Shiga-like toxin (SLT) is a historical term for similar or identical toxins produced by Escherichia coli. The most common sources for Shiga toxin are the bacteria S. dysenteriae and some serotypes of Escherichia coli (STEC), which includes serotypes O157:H7, and O104:H4.

Heme oxygenase, or haem oxygenase, is an enzyme that catalyzes the degradation of heme to produce biliverdin, ferrous ion, and carbon monoxide.

Succinyl coenzyme A synthetase is an enzyme that catalyzes the reversible reaction of succinyl-CoA to succinate. The enzyme facilitates the coupling of this reaction to the formation of a nucleoside triphosphate molecule from an inorganic phosphate molecule and a nucleoside diphosphate molecule. It plays a key role as one of the catalysts involved in the citric acid cycle, a central pathway in cellular metabolism, and it is located within the mitochondrial matrix of a cell.
AlkB is a protein found in E. coli, induced during an adaptive response and involved in the direct reversal of alkylation damage. AlkB specifically removes alkylation damage to single stranded (SS) DNA caused by SN2 type of chemical agents. It efficiently removes methyl groups from 1-methyl adenines, 3-methyl cytosines in SS DNA. AlkB is an alpha-ketoglutarate-dependent hydroxylase, a superfamily non-haem iron-containing proteins. It oxidatively demethylates the DNA substrate. Demethylation by AlkB is accompanied with release of CO2, succinate, and formaldehyde.
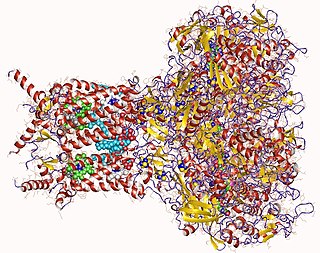
Formate dehydrogenases are a set of enzymes that catalyse the oxidation of formate to carbon dioxide, donating the electrons to a second substrate, such as NAD+ in formate:NAD+ oxidoreductase (EC 1.17.1.9) or to a cytochrome in formate:ferricytochrome-b1 oxidoreductase (EC 1.2.2.1). This family of enzymes has attracted attention as inspiration or guidance on methods for the carbon dioxide fixation, relevant to global warming.
Ubiquinol oxidases are enzymes in the bacterial electron transport chain that oxidise ubiquinol into ubiquinone and reduce oxygen to water. These enzymes are one set of the many alternative terminal oxidases in the branched prokaryotic electron transport chain. The overall structure of the E. coli ubiquinol oxidase is similar to that of the mammalian Cytochrome c oxidase, with the addition of a polar ubiquinol-binding site embedded in the membrane.

Nitric oxide dioxygenase (EC 1.14.12.17) is an enzyme that catalyzes the conversion of nitric oxide (NO) to nitrate (NO−
3) . The net reaction for the reaction catalyzed by nitric oxide dioxygenase is shown below:

Cytochrome c nitrite reductase (ccNiR) is a bacterial enzyme that catalyzes the six electron reduction of nitrite to ammonia; an important step in the biological nitrogen cycle. The enzyme catalyses the second step in the two step conversion of nitrate to ammonia, which allows certain bacteria to use nitrite as a terminal electron acceptor, rather than oxygen, during anaerobic conditions. During this process, ccNiR draws electrons from the quinol pool, which are ultimately provided by a dehydrogenase such as formate dehydrogenase or hydrogenase. These dehydrogenases are responsible for generating a proton motive force.

Methionine aminopeptidase 2 is an enzyme that in humans is encoded by the METAP2 gene.

In enzymology, a serine O-acetyltransferase is an enzyme that catalyzes the chemical reaction
Shigatoxigenic Escherichia coli (STEC) and verotoxigenic E. coli (VTEC) are strains of the bacterium Escherichia coli that produce Shiga toxin. Only a minority of the strains cause illness in humans. The ones that do are collectively known as enterohemorrhagic E. coli (EHEC) and are major causes of foodborne illness. When infecting the large intestine of humans, they often cause gastroenteritis, enterocolitis, and bloody diarrhea and sometimes cause a severe complication called hemolytic-uremic syndrome (HUS). Cattle is an important natural reservoir for EHEC because the colonised adult ruminants are asymptomatic. This is because they lack vascular expression of the target receptor for Shiga toxins. The group and its subgroups are known by various names. They are distinguished from other strains of intestinal pathogenic E. coli including enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), and diffusely adherent E. coli (DAEC).

Escherichia coli is a gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms (endotherms). Most E. coli strains are harmless, but pathogenic varieties cause serious food poisoning, septic shock, meningitis, or urinary tract infections in humans. Unlike normal flora E. coli, the pathogenic varieties produce toxins and other virulence factors that enable them to reside in parts of the body normally not inhabited by E. coli, and to damage host cells. These pathogenic traits are encoded by virulence genes carried only by the pathogens.
Formate dehydrogenase-N (EC 1.1.5.6, Fdh-N, FdnGHI, nitrate-inducible formate dehydrogenase, formate dehydrogenase N, FDH-N, nitrate inducible Fdn, nitrate inducible formate dehydrogenase) is an enzyme with systematic name formate:quinone oxidoreductase. This enzyme catalyses the following chemical reaction
Nitrate reductase (quinone) (EC 1.7.5.1, nitrate reductase A, nitrate reductase Z, quinol/nitrate oxidoreductase, quinol-nitrate oxidoreductase, quinol:nitrate oxidoreductase, NarA, NarZ, NarGHI) is an enzyme with systematic name nitrite:quinone oxidoreductase. This enzyme catalyses the following chemical reaction
Ubiquinol oxidase (H+-transporting) (EC 7.1.1.3, cytochrome bb3 oxidase, cytochrome bo oxidase, cytochrome bd-I oxidase) is an enzyme with systematic name ubiquinol:O2 oxidoreductase (H+-transporting). This enzyme catalyses the following chemical reaction

Cytochrome d, previously known as cytochrome a2, is a name for all cytochromes that contain heme D as a cofactor. Two unrelated classes of cytochrome d are known: Cytochrome bd, an enzyme that generates a charge across the membrane so that protons will move, and cytochrome cd1, a nitrite reductase.














