| |
 | |
| Names | |
|---|---|
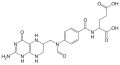
| IUPAC name N-[4-({[(6Ξ)-2-Amino-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl]methyl}amino)benzoyl]-L-glutamic acid | |
| Systematic IUPAC name (2S)-2-[4-({[(6Ξ)-2-Amino-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl]methyl}amino)benzamido]pentanedioic acid | |
| Identifiers | |
3D model (JSmol) | |
| 101189 | |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
| MeSH | 5,6,7,8-tetrahydrofolic+acid |
PubChem CID | |
| UNII | |
| |
| |
| Properties | |
| C19H23N7O6 | |
| Molar mass | 445.43 g/mol |
| Melting point | 250 °C (482 °F; 523 K) |
| 0.27 g/L | |
| Acidity (pKa) | 3.51 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Tetrahydrofolic acid (THFA), or tetrahydrofolate, is a folic acid derivative.