In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of a molecule. Chemical shifts are also used to describe signals in other forms of spectroscopy such as photoemission spectroscopy.
CIDNP, often pronounced like "kidnip", is a nuclear magnetic resonance (NMR) technique that is used to study chemical reactions that involve radicals. It detects the non-Boltzmann (non-thermal) nuclear spin state distribution produced in these reactions as enhanced absorption or emission signals.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic field. This re-orientation occurs with absorption of electromagnetic radiation in the radio frequency region from roughly 4 to 900 MHz, which depends on the isotopic nature of the nucleus and increased proportionally to the strength of the external magnetic field. Notably, the resonance frequency of each NMR-active nucleus depends on its chemical environment. As a result, NMR spectra provide information about individual functional groups present in the sample, as well about connections between nearby nuclei in the same molecule. As the NMR spectra are unique or highly characteristic to individual compounds and functional groups, NMR spectroscopy is one of the most important methods to identify molecular structures, particularly of organic compounds.

Solid-state NMR (ssNMR) spectroscopy is a technique for characterizing atomic level structure in solid materials e.g. powders, single crystals and amorphous samples and tissues using nuclear magnetic resonance (NMR) spectroscopy. The anisotropic part of many spin interactions are present in solid-state NMR, unlike in solution-state NMR where rapid tumbling motion averages out many of the spin interactions. As a result, solid-state NMR spectra are characterised by larger linewidths than in solution state NMR, which can be utilized to give quantitative information on the molecular structure, conformation and dynamics of the material. Solid-state NMR is often combined with magic angle spinning to remove anisotropic interactions and improve the resolution as well as the sensitivity of the technique.

Martin Karplus is an Austrian and American theoretical chemist. He is the Director of the Biophysical Chemistry Laboratory, a joint laboratory between the French National Center for Scientific Research and the University of Strasbourg, France. He is also the Theodore William Richards Professor of Chemistry, emeritus at Harvard University. Karplus received the 2013 Nobel Prize in Chemistry, together with Michael Levitt and Arieh Warshel, for "the development of multiscale models for complex chemical systems".

Nuclear magnetic resonance quantum computing (NMRQC) is one of the several proposed approaches for constructing a quantum computer, that uses the spin states of nuclei within molecules as qubits. The quantum states are probed through the nuclear magnetic resonances, allowing the system to be implemented as a variation of nuclear magnetic resonance spectroscopy. NMR differs from other implementations of quantum computers in that it uses an ensemble of systems, in this case molecules, rather than a single pure state.
Peter John Hore is a British chemist and academic. He is a Professor of Chemistry at the University of Oxford and fellow of Corpus Christi College, Oxford. He is the author of two Oxford Chemistry Primers on Nuclear Magnetic Resonance (NMR) and research articles primarily in the area of NMR, electron paramagnetic resonance (EPR), spin chemistry and magnetoreception during bird migration.
In nuclear chemistry and nuclear physics, J-couplings are mediated through chemical bonds connecting two spins. It is an indirect interaction between two nuclear spins that arises from hyperfine interactions between the nuclei and local electrons. In NMR spectroscopy, J-coupling contains information about relative bond distances and angles. Most importantly, J-coupling provides information on the connectivity of chemical bonds. It is responsible for the often complex splitting of resonance lines in the NMR spectra of fairly simple molecules.

The residual dipolar coupling between two spins in a molecule occurs if the molecules in solution exhibit a partial alignment leading to an incomplete averaging of spatially anisotropic dipolar couplings.
Herbert Sander Gutowsky was an American chemist who was a professor of chemistry at the University of Illinois Urbana-Champaign. Gutowsky was the first to apply nuclear magnetic resonance (NMR) methods to the field of chemistry. He used nuclear magnetic resonance spectroscopy to determine the structure of molecules. His pioneering work developed experimental control of NMR as a scientific instrument, connected experimental observations with theoretical models, and made NMR one of the most effective analytical tools for analysis of molecular structure and dynamics in liquids, solids, and gases, used in chemical and medical research, His work was relevant to the solving of problems in chemistry, biochemistry, and materials science, and has influenced many of the subfields of more recent NMR spectroscopy.
Carbon satellites in physics and spectroscopy, are small peaks that can be seen shouldering the main peaks in the nuclear magnetic resonance (NMR) spectrum. These peaks can occur in the NMR spectrum of any NMR active atom where those atoms adjoin a carbon atom. However, Carbon satellites are most often encountered in proton NMR.
Raymond Freeman FRS was a British chemist and professor at Jesus College, Cambridge who made important contributions to NMR spectroscopy.

Fluorine-19 nuclear magnetic resonance spectroscopy is an analytical technique used to detect and identify fluorine-containing compounds. 19F is an important nucleus for NMR spectroscopy because of its receptivity and large chemical shift dispersion, which is greater than that for proton nuclear magnetic resonance spectroscopy.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI). The original application of NMR to condensed matter physics is nowadays mostly devoted to strongly correlated electron systems. It reveals large manybody couplings by fast broadband detection and it should not to be confused with solid state NMR, which aims at removing the effect of the same couplings by Magic Angle Spinning techniques.
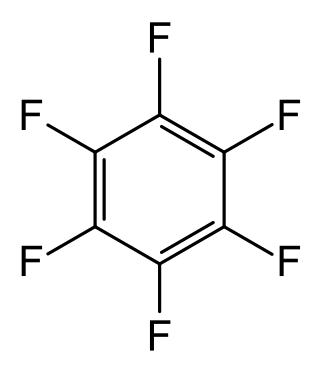
Hexafluorobenzene, HFB, C
6F
6, or perfluorobenzene is an organofluorine compound. In this derivative of benzene, all hydrogen atoms have been replaced by fluorine atoms. The technical uses of the compound are limited, although it has some specialized uses in the laboratory owing to distinctive spectroscopic properties.
Nucleic acid NMR is the use of nuclear magnetic resonance spectroscopy to obtain information about the structure and dynamics of nucleic acid molecules, such as DNA or RNA. It is useful for molecules of up to 100 nucleotides, and as of 2003, nearly half of all known RNA structures had been determined by NMR spectroscopy.
Malcolm Harris Levitt is a British physical chemist and nuclear magnetic resonance (NMR) spectroscopist. He is Professor in Physical Chemistry at the University of Southampton and was elected a Fellow of the Royal Society in 2007.
Christiane Renate Timmel is a German chemist who is Director of the Centre for Advanced Electron Spin Resonance at the University of Oxford. Her group make use of electron-spin resonance to understand long-range structures in chemical and biological systems. Timmel was awarded the Tilden Prize on 2020 by the Royal Society of Chemistry for her contributions to electron spin resonance.

Alfred G. Redfield was an American physicist and biochemist. In 1955 he published the Redfield relaxation theory, effectively moving the practice of NMR or Nuclear magnetic resonance from the realm of classical physics to the realm of semiclassical physics. He continued to find novel magnetic resonance applications to solve real-world problems throughout his life.

Spinach is an open-source magnetic resonance simulation package initially released in 2011 and continuously updated since. The package is written in Matlab and makes use of the built-in parallel computing and GPU interfaces of Matlab.










