
Radiation therapy or radiotherapy is a treatment using ionizing radiation, generally provided as part of cancer therapy to either kill or control the growth of malignant cells. It is normally delivered by a linear particle accelerator. Radiation therapy may be curative in a number of types of cancer if they are localized to one area of the body, and have not spread to other parts. It may also be used as part of adjuvant therapy, to prevent tumor recurrence after surgery to remove a primary malignant tumor. Radiation therapy is synergistic with chemotherapy, and has been used before, during, and after chemotherapy in susceptible cancers. The subspecialty of oncology concerned with radiotherapy is called radiation oncology. A physician who practices in this subspecialty is a radiation oncologist.

External beam radiation therapy (EBRT) is a form of radiotherapy that utilizes a high-energy collimated beam of ionizing radiation, from a source outside the body, to target and kill cancer cells. A radiotherapy beam is composed of particles which travel in a consistent direction; each radiotherapy beam consists of one type of particle intended for use in treatment, though most beams contain some contamination by other particle types.

Brachytherapy is a form of radiation therapy where a sealed radiation source is placed inside or next to the area requiring treatment. The word "brachytherapy" comes from the Greek word βραχύς, brachys, meaning "short-distance" or "short". Brachytherapy is commonly used as an effective treatment for cervical, prostate, breast, esophageal and skin cancer and can also be used to treat tumours in many other body sites. Treatment results have demonstrated that the cancer-cure rates of brachytherapy are either comparable to surgery and external beam radiotherapy (EBRT) or are improved when used in combination with these techniques. Brachytherapy can be used alone or in combination with other therapies such as surgery, EBRT and chemotherapy.

Megavoltage X-rays are produced by linear accelerators ("linacs") operating at voltages in excess of 1000 kV (1 MV) range, and therefore have an energy in the MeV range. The voltage in this case refers to the voltage used to accelerate electrons in the linear accelerator and indicates the maximum possible energy of the photons which are subsequently produced. They are used in medicine in external beam radiotherapy to treat neoplasms, cancer and tumors. Beams with a voltage range of 4-25 MV are used to treat deeply buried cancers because radiation oncologists find that they penetrate well to deep sites within the body. Lower energy x-rays, called orthovoltage X-rays, are used to treat cancers closer to the surface.

In medicine, proton therapy, or proton radiotherapy, is a type of particle therapy that uses a beam of protons to irradiate diseased tissue, most often to treat cancer. The chief advantage of proton therapy over other types of external beam radiotherapy is that the dose of protons is deposited over a narrow range of depth; hence in minimal entry, exit, or scattered radiation dose to healthy nearby tissues.

Radiosurgery is surgery using radiation, that is, the destruction of precisely selected areas of tissue using ionizing radiation rather than excision with a blade. Like other forms of radiation therapy, it is usually used to treat cancer. Radiosurgery was originally defined by the Swedish neurosurgeon Lars Leksell as "a single high dose fraction of radiation, stereotactically directed to an intracranial region of interest".
Vaginal cancer is an extraordinarily rare form of cancer that develops in the tissue of the vagina. Primary vaginal cancer originates from the vaginal tissue – most frequently squamous cell carcinoma, but primary vaginal adenocarcinoma, sarcoma, and melanoma have also been reported – while secondary vaginal cancer involves the metastasis of a cancer that originated in a different part of the body. Secondary vaginal cancer is more common. Signs of vaginal cancer may include abnormal vaginal bleeding, dysuria, tenesmus, or pelvic pain, though as many as 20% of women diagnosed with vaginal cancer are asymptomatic at the time of diagnosis. Vaginal cancer occurs more frequently in women over age 50, and the mean age of diagnosis of vaginal cancer is 60 years. It often can be cured if found and treated in early stages. Surgery alone or surgery combined with pelvic radiation is typically used to treat vaginal cancer.

Fast neutron therapy utilizes high energy neutrons typically between 50 and 70 MeV to treat cancer. Most fast neutron therapy beams are produced by reactors, cyclotrons (d+Be) and linear accelerators. Neutron therapy is currently available in Germany, Russia, South Africa and the United States. In the United States, one treatment center is operational, in Seattle, Washington. The Seattle center uses a cyclotron which produces a proton beam impinging upon a beryllium target.

In radiotherapy, radiation treatment planning (RTP) is the process in which a team consisting of radiation oncologists, radiation therapist, medical physicists and medical dosimetrists plan the appropriate external beam radiotherapy or internal brachytherapy treatment technique for a patient with cancer.
Electron therapy or electron beam therapy (EBT) is a kind of external beam radiotherapy where electrons are directed to a tumor site for medical treatment of cancer.

Tomotherapy is a type of radiation therapy treatment machine. In tomotherapy a thin radiation beam is modulated as it rotates around the patient, while they are moved through the bore of the machine. The name comes from the use of a strip-shaped beam, so that only one “slice” of the target is exposed at any one time by the radiation. The external appearance of the system and movement of the radiation source and patient can be considered analogous to a CT scanner, which uses lower doses of radiation for imaging. Like a conventional machine used for X-ray external beam radiotherapy, it [the tomotherapy machine] generates the radiation beam, but the external appearance of the machine, patient positioning, and treatment delivery differ. Conventional linacs do not work on a slice-by-slice basis but typically have a large area beam which can also be resized and modulated.

Cobalt therapy is the medical use of gamma rays from the radioisotope cobalt-60 to treat conditions such as cancer. Beginning in the 1950s, cobalt-60 was widely used in external beam radiotherapy (teletherapy) machines, which produced a beam of gamma rays which was directed into the patient's body to kill tumor tissue. Because these "cobalt machines" were expensive and required specialist support, they were often housed in cobalt units. Cobalt therapy was a revolutionary advance in radiotherapy in the post-World War II period but is now being replaced by other technologies such as linear accelerators.
Particle therapy is a form of external beam radiotherapy using beams of energetic neutrons, protons, or other heavier positive ions for cancer treatment. The most common type of particle therapy as of August 2021 is proton therapy.
Intraoperative electron radiation therapy is the application of electron radiation directly to the residual tumor or tumor bed during cancer surgery. Electron beams are useful for intraoperative radiation treatment because, depending on the electron energy, the dose falls off rapidly behind the target site, therefore sparing underlying healthy tissue.
Breast cancer management takes different approaches depending on physical and biological characteristics of the disease, as well as the age, over-all health and personal preferences of the patient. Treatment types can be classified into local therapy and systemic treatment. Local therapy is most efficacious in early stage breast cancer, while systemic therapy is generally justified in advanced and metastatic disease, or in diseases with specific phenotypes.

Brachytherapy is a type of radiotherapy, or radiation treatment, offered to certain cancer patients. There are two types of brachytherapy – high dose-rate (HDR) and low dose-rate (LDR). LDR brachytherapy is the one most commonly used to treat prostate cancer. It may be referred to as 'seed implantation' or it may be called 'pinhole surgery'.

Targeted intra-operative radiotherapy, also known as targeted IORT, is a technique of giving radiotherapy to the tissues surrounding a cancer after its surgical removal, a form of intraoperative radiation therapy. The technique was designed in 1998 at the University College London.
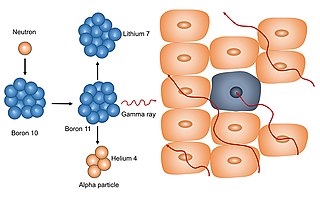
Neutron capture therapy (NCT) is a type of radiotherapy for treating locally invasive malignant tumors such as primary brain tumors, recurrent cancers of the head and neck region, and cutaneous and extracutaneous melanomas. It is a two-step process: first, the patient is injected with a tumor-localizing drug containing the stable isotope boron-10 (10B), which has a high propensity to capture low energy "thermal" neutrons. The neutron cross section of 10B is 1,000 times more than that of other elements, such as nitrogen, hydrogen, or oxygen, that occur in tissue. In the second step, the patient is radiated with epithermal neutrons, the sources of which in the past have been nuclear reactors and now are accelerators that produce higher energy epithermal neutrons. After losing energy as they penetrate tissue, the resultant low energy "thermal" neutrons are captured by the 10B atoms. The resulting decay reaction yields high-energy alpha particles that kill the cancer cells that have taken up enough 10B.
Docrates Cancer Center is the first and currently the only private hospital in the Nordic countries that comprehensively specialises in cancer treatment. It operates in Helsinki, Finland. It characterises its operations as those complementing the public sector. Docrates Oy was established in 2006 and the hospital started its operations at the premises of Eira Hospital in autumn 2007. It moved to its own premises in Jätkäsaari, Helsinki, in 2009, where it has hospital rights. There is a ward and Health and Recovery Center located at Docrates Cancer Center. Among other things, diagnostics, pharmacotherapy, radiation therapy and isotopic treatments are carried out at the hospital. Cancer surgeries are performed in partner hospitals. Docrates also participates in clinical trials and the testing and development of new treatments.

Jayant S. Vaidya is a British-Indian surgeon-oncologist and clinical academic who, together with Michael Baum and Jeffrey Tobias, developed the technique called targeted intra-operative radiotherapy (TARGIT). He is a professor of surgery and oncology at the University College London, London and the author of two books on breast cancer, one on tobacco eradication, and over 200 academic articles.












