
Hydrogen is a chemical element; it has symbol H and atomic number 1. It is the lightest element and, at standard conditions, is a gas of diatomic molecules with the formula H2, sometimes called dihydrogen, but more commonly called hydrogen gas, molecular hydrogen or simply hydrogen. It is colorless, odorless, tasteless, non-toxic, and highly combustible. Constituting approximately 75% of all normal matter, hydrogen is the most abundant chemical substance in the universe. Stars, including the Sun, primarily consist of hydrogen in a plasma state, while on Earth, hydrogen is found in water, organic compounds, as dihydrogen, and in other molecular forms. The most common isotope of hydrogen consists of one proton, one electron, and no neutrons.

In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen atom with two electrons. The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are also called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed.
Borderline hydrides typically refer to hydrides formed of hydrogen and elements of the periodic table in group 11 and group 12 and indium (In) and thallium (Tl). These compounds have properties intermediate between covalent hydrides and saline hydrides. Hydrides are chemical compounds that contain a metal and hydrogen acting as a negative ion.
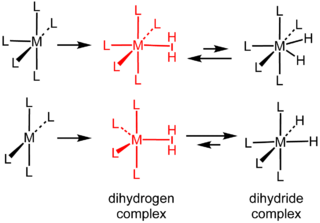
Dihydrogen complexes are coordination complexes containing intact H2 as a ligand. They are a subset of sigma complexes. The prototypical complex is W(CO)3(PCy3)2(H2). This class of compounds represent intermediates in metal-catalyzed reactions involving hydrogen. Hundreds of dihydrogen complexes have been reported. Most examples are cationic transition metals complexes with octahedral geometry.
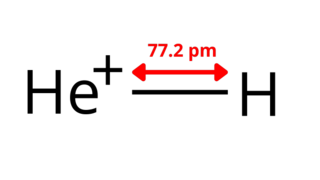
The helium hydride ion, hydridohelium(1+) ion, or helonium is a cation (positively charged ion) with chemical formula HeH+. It consists of a helium atom bonded to a hydrogen atom, with one electron removed. It can also be viewed as protonated helium. It is the lightest heteronuclear ion, and is believed to be the first compound formed in the Universe after the Big Bang.
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions. The term "hydride" is used loosely: some of them are acidic (e.g., H2Fe(CO)4), whereas some others are hydridic, having H−-like character (e.g., ZnH2).

Magnesium hydride is the chemical compound with the molecular formula MgH2. It contains 7.66% by weight of hydrogen and has been studied as a potential hydrogen storage medium.
Zinc hydride is an inorganic compound with the chemical formula ZnH2. It is a white, odourless solid which slowly decomposes into its elements at room temperature; despite this it is the most stable of the binary first row transition metal hydrides. A variety of coordination compounds containing Zn–H bonds are used as reducing agents, but ZnH2 itself has no common applications.
Binary compounds of hydrogen are binary chemical compounds containing just hydrogen and one other chemical element. By convention all binary hydrogen compounds are called hydrides even when the hydrogen atom in it is not an anion. These hydrogen compounds can be grouped into several types.
Cadmium hydride is an inorganic compound with the chemical formula (CdH
2)
n. It is a solid, known only as a thermally unstable, insoluble white powder.

Mercury(II) hydride is an inorganic compound with the chemical formula HgH
2. It is both thermodynamically and kinetically unstable at ambient temperature, and as such, little is known about its bulk properties. However, it known as a white, crystalline solid, which is kinetically stable at temperatures below −125 °C (−193 °F), which was synthesised for the first time in 1951.

Chromium(I) hydride, systematically named chromium hydride, is an inorganic compound with the chemical formula (CrH)
n. It occurs naturally in some kinds of stars where it has been detected by its spectrum. However, molecular chromium(I) hydride with the formula CrH has been isolated in solid gas matrices. The molecular hydride is very reactive. As such the compound is not well characterised, although many of its properties have been calculated via computational chemistry.
Chromium(II) hydride, systematically named chromium dihydride and poly(dihydridochromium) is pale brown solid inorganic compound with the chemical formula (CrH2)n. Although it is thermodynamically unstable toward decomposition at ambient temperatures, it is kinetically metastable.

Iron(I) hydride, systematically named iron hydride and poly(hydridoiron) is a solid inorganic compound with the chemical formula (FeH)
n (also written ([FeH])
n or FeH). It is both thermodynamically and kinetically unstable toward decomposition at ambient temperature, and as such, little is known about its bulk properties.
Iron(II) hydride, systematically named iron dihydride and poly(dihydridoiron) is solid inorganic compound with the chemical formula (FeH
2)
n (also written ([FeH
2])n or FeH
2). ). It is kinetically unstable at ambient temperature, and as such, little is known about its bulk properties. However, it is known as a black, amorphous powder, which was synthesised for the first time in 2014.

Iron–hydrogen alloy, also known as iron hydride, is an alloy of iron and hydrogen and other elements. Because of its lability when removed from a hydrogen atmosphere, it has no uses as a structural material.
Borane, also known as borine, is an unstable and highly reactive molecule with the chemical formula BH
3. The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated the likely existence of the borane molecule. However, the molecular species BH3 is a very strong Lewis acid. Consequently, it is highly reactive and can only be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen. It normally dimerizes to diborane in the absence of other chemicals.

Magnesium monohydride is a molecular gas with formula MgH that exists at high temperatures, such as the atmospheres of the Sun and stars. It was originally known as magnesium hydride, although that name is now more commonly used when referring to the similar chemical magnesium dihydride.
Neon compounds are chemical compounds containing the element neon (Ne) with other molecules or elements from the periodic table. Compounds of the noble gas neon were believed not to exist, but there are now known to be molecular ions containing neon, as well as temporary excited neon-containing molecules called excimers. Several neutral neon molecules have also been predicted to be stable, but are yet to be discovered in nature. Neon has been shown to crystallize with other substances and form clathrates or Van der Waals solids.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.










