
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.

Epitaxy refers to a type of crystal growth or material deposition in which new crystalline layers are formed with one or more well-defined orientations with respect to the crystalline seed layer. The deposited crystalline film is called an epitaxial film or epitaxial layer. The relative orientation(s) of the epitaxial layer to the seed layer is defined in terms of the orientation of the crystal lattice of each material. For most epitaxial growths, the new layer is usually crystalline and each crystallographic domain of the overlayer must have a well-defined orientation relative to the substrate crystal structure. Epitaxy can involve single-crystal structures, although grain-to-grain epitaxy has been observed in granular films. For most technological applications, single-domain epitaxy, which is the growth of an overlayer crystal with one well-defined orientation with respect to the substrate crystal, is preferred. Epitaxy can also play an important role in the growth of superlattice structures.

Molecular-beam epitaxy (MBE) is an epitaxy method for thin-film deposition of single crystals. MBE is widely used in the manufacture of semiconductor devices, including transistors. MBE is used to make diodes and MOSFETs at microwave frequencies, and to manufacture the lasers used to read optical discs.
SiGe, or silicon–germanium, is an alloy with any molar ratio of silicon and germanium, i.e. with a molecular formula of the form Si1−xGex. It is commonly used as a semiconductor material in integrated circuits (ICs) for heterojunction bipolar transistors or as a strain-inducing layer for CMOS transistors. IBM introduced the technology into mainstream manufacturing in 1989. This relatively new technology offers opportunities in mixed-signal circuit and analog circuit IC design and manufacture. SiGe is also used as a thermoelectric material for high-temperature applications (>700 K).

Strained silicon is a layer of silicon in which the silicon atoms are stretched beyond their normal interatomic distance. This can be accomplished by putting the layer of silicon over a substrate of silicon–germanium. As the atoms in the silicon layer align with the atoms of the underlying silicon germanium layer, the links between the silicon atoms become stretched, thereby leading to strained silicon. Moving these silicon atoms further apart reduces the atomic forces that interfere with the movement of electrons through the transistors and thus improved mobility, resulting in better chip performance and lower energy consumption. These electrons can move 70% faster allowing strained silicon transistors to switch 35% faster.

Metalorganic vapour-phase epitaxy (MOVPE), also known as organometallic vapour-phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method used to produce single- or polycrystalline thin films. It is a process for growing crystalline layers to create complex semiconductor multilayer structures. In contrast to molecular-beam epitaxy (MBE), the growth of crystals is by chemical reaction and not physical deposition. This takes place not in vacuum, but from the gas phase at moderate pressures. As such, this technique is preferred for the formation of devices incorporating thermodynamically metastable alloys, and it has become a major process in the manufacture of optoelectronics, such as light-emitting diodes, its most widespread application. It was first demonstrated in 1967 at North American Aviation Autonetics Division in Anaheim CA by Harold M. Manasevit.

Germane is the chemical compound with the formula GeH4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water. Germane is a group 14 hydride.
Chemical beam epitaxy (CBE) forms an important class of deposition techniques for semiconductor layer systems, especially III-V semiconductor systems. This form of epitaxial growth is performed in an ultrahigh vacuum system. The reactants are in the form of molecular beams of reactive gases, typically as the hydride or a metalorganic. The term CBE is often used interchangeably with metal-organic molecular beam epitaxy (MOMBE). The nomenclature does differentiate between the two processes, however. When used in the strictest sense, CBE refers to the technique in which both components are obtained from gaseous sources, while MOMBE refers to the technique in which the group III component is obtained from a gaseous source and the group V component from a solid source.
GeSbTe (germanium-antimony-tellurium or GST) is a phase-change material from the group of chalcogenide glasses used in rewritable optical discs and phase-change memory applications. Its recrystallization time is 20 nanoseconds, allowing bitrates of up to 35 Mbit/s to be written and direct overwrite capability up to 106 cycles. It is suitable for land-groove recording formats. It is often used in rewritable DVDs. New phase-change memories are possible using n-doped GeSbTe semiconductor. The melting point of the alloy is about 600 °C (900 K) and the crystallization temperature is between 100 and 150 °C.
Organogermanium chemistry is the science of chemical species containing one or more C–Ge bonds. Germanium shares group 14 in the periodic table with carbon, silicon, tin and lead. Historically, organogermanes are considered as nucleophiles and the reactivity of them is between that of organosilicon and organotin compounds. Some organogermanes have enhanced reactivity compared with their organosilicon and organoboron analogues in some cross-coupling reactions.

Trimethylindium, often abbreviated to TMI or TMIn, is the organoindium compound with the formula In(CH3)3. It is a colorless, pyrophoric solid. Unlike trimethylaluminium, but akin to trimethylgallium, TMI is monomeric.

Trimethylgallium, often abbreviated to TMG or TMGa, is the organogallium compound with the formula Ga(CH3)3. It is a colorless, pyrophoric liquid. Unlike trimethylaluminium, TMG adopts a monomeric structure. When examined in detail, the monomeric units are clearly linked by multiple weak Ga---C interactions, reminiscent of the situation for trimethylindium.
Dr. Harold M. Manasevit (1927–2008) was an American materials scientist.
Selective area epitaxy is the local growth of epitaxial layer through a patterned amorphous dielectric mask (typically SiO2 or Si3N4) deposited on a semiconductor substrate. Semiconductor growth conditions are selected to ensure epitaxial growth on the exposed substrate, but not on the dielectric mask. SAE can be executed in various epitaxial growth methods such as molecular beam epitaxy (MBE), metalorganic vapour phase epitaxy (MOVPE) and chemical beam epitaxy (CBE). By SAE, semiconductor nanostructures such as quantum dots and nanowires can be grown to their designed places.
A Bubbler cylinder is a component of a unit for the metal organic chemical vapor deposition (MOCVD). They are devices that are used for conveying electronic grade metalorganic compounds from a liquid or solid precursor into a usable vapor.

Triethylgallium is the organogallium compound with the formul Ga(C2H5)3. Also called TEGa, it is a metalorganic source of gallium for metalorganic vapour phase epitaxy (MOVPE) of compound semiconductors. It is a colorless pyrophoric liquid, typically handled with air-free techniques. It was discovered by Cornell University chemists L. M. Dennis and Winton Patnode in 1931.
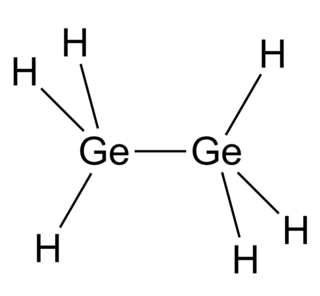
Digermane is an inorganic compound with the chemical formula Ge2H6. One of the few hydrides of germanium, it is a colourless liquid. Its molecular geometry is similar to ethane.

Low-energy plasma-enhanced chemical vapor deposition (LEPECVD) is a plasma-enhanced chemical vapor deposition technique used for the epitaxial deposition of thin semiconductor films. A remote low energy, high density DC argon plasma is employed to efficiently decompose the gas phase precursors while leaving the epitaxial layer undamaged, resulting in high quality epilayers and high deposition rates.
Germanium compounds are chemical compounds formed by the element germanium (Ge). Germanium is insoluble in dilute acids and alkalis but dissolves slowly in hot concentrated sulfuric and nitric acids and reacts violently with molten alkalis to produce germanates ([GeO
3]2−
). Germanium occurs mostly in the oxidation state +4 although many +2 compounds are known. Other oxidation states are rare: +3 is found in compounds such as Ge2Cl6, and +3 and +1 are found on the surface of oxides, or negative oxidation states in germanides, such as −4 in Mg
2Ge. Germanium cluster anions (Zintl ions) such as Ge42−, Ge94−, Ge92−, [(Ge9)2]6− have been prepared by the extraction from alloys containing alkali metals and germanium in liquid ammonia in the presence of ethylenediamine or a cryptand. The oxidation states of the element in these ions are not integers—similar to the ozonides O3−.
Joan M. Redwing is an American materials scientist known for research on electronic and optoelectronic materials, including the processing of semiconductor thin films and nanomaterials by metalorganic chemical vapor deposition (MOCVD). Redwing is a distinguished professor of materials science and engineering and electrical engineering at Pennsylvania State University and director of the university's 2D Crystal Consortium research facility. She is a fellow of the American Association for the Advancement of Science, the American Physical Society, and the Materials Research Society.













