
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point. At the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts to become a liquid.

Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in its native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.
A metalloid is a chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity, the term remains in use in the literature.

Freezing is a phase transition in which a liquid turns into a solid when its temperature is lowered below its freezing point. In accordance with the internationally established definition, freezing means the solidification phase change of a liquid or the liquid content of a substance, usually due to cooling.
Phase-change memory is a type of non-volatile random-access memory. PRAMs exploit the unique behaviour of chalcogenide glass. In PCM, heat produced by the passage of an electric current through a heating element generally made of titanium nitride is used to either quickly heat and quench the glass, making it amorphous, or to hold it in its crystallization temperature range for some time, thereby switching it to a crystalline state. PCM also has the ability to achieve a number of distinct intermediary states, thereby having the ability to hold multiple bits in a single cell, but the difficulties in programming cells in this way has prevented these capabilities from being implemented in other technologies with the same capability.

Crystallization is the process by which solids form, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, cooling rate, and in the case of liquid crystals, time of fluid evaporation.
Chalcogenide glass is a glass containing one or more chalcogens. Polonium is also a chalcogen but is not used because of its strong radioactivity. Chalcogenide materials behave rather differently from oxides, in particular their lower band gaps contribute to very dissimilar optical and electrical properties.

In materials science, a single crystal is a material in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample, with no grain boundaries. The absence of the defects associated with grain boundaries can give monocrystals unique properties, particularly mechanical, optical and electrical, which can also be anisotropic, depending on the type of crystallographic structure. These properties, in addition to making some gems precious, are industrially used in technological applications, especially in optics and electronics.

Metalorganic vapour-phase epitaxy (MOVPE), also known as organometallic vapour-phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method used to produce single- or polycrystalline thin films. It is a process for growing crystalline layers to create complex semiconductor multilayer structures. In contrast to molecular-beam epitaxy (MBE), the growth of crystals is by chemical reaction and not physical deposition. This takes place not in vacuum, but from the gas phase at moderate pressures. As such, this technique is preferred for the formation of devices incorporating thermodynamically metastable alloys, and it has become a major process in the manufacture of optoelectronics, such as light-emitting diodes, its most widespread application. It was first demonstrated in 1967 at North American Aviation Autonetics Division in Anaheim CA by Harold M. Manasevit.
AgInSbTe, or silver-indium-antimony-tellurium, is a phase change material from the group of chalcogenide glasses, used in rewritable optical discs and phase-change memory applications. It is a quaternary compound of silver, indium, antimony, and tellurium.

Polyamorphism is the ability of a substance to exist in several different amorphous modifications. It is analogous to the polymorphism of crystalline materials. Many amorphous substances can exist with different amorphous characteristics. However, polyamorphism requires two distinct amorphous states with a clear, discontinuous (first-order) phase transition between them. When such a transition occurs between two stable liquid states, a polyamorphic transition may also be referred to as a liquid–liquid phase transition.

Germanium telluride (GeTe) is a chemical compound of germanium and tellurium and is a component of chalcogenide glass. It shows semimetallic conduction and ferroelectric behaviour.

Tin selenide, also known as stannous selenide, is an inorganic compound with the formula SnSe. Tin(II) selenide is a typical layered metal chalcogenide as it includes a group 16 anion (Se2−) and an electropositive element (Sn2+), and is arranged in a layered structure. Tin(II) selenide is a narrow band-gap (IV-VI) semiconductor structurally analogous to black phosphorus. It has received considerable interest for applications including low-cost photovoltaics, and memory-switching devices.
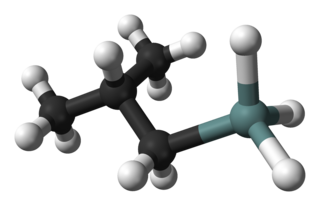
Isobutylgermane (IBGe, Chemical formula: (CH3)2CHCH2GeH3, is an organogermanium compound. It is a colourless, volatile liquid that is used in MOVPE (Metalorganic Vapor Phase Epitaxy) as an alternative to germane. IBGe is used in the deposition of Ge films and Ge-containing thin semiconductor films such as SiGe in strained silicon application, and GeSbTe in NAND Flash applications.

Antimony telluride is an inorganic compound with the chemical formula Sb2Te3. As is true of other pnictogen chalcogenide layered materials, it is a grey crystalline solid with layered structure. Layers consist of two atomic sheets of antimony and three atomic sheets of tellurium and are held together by weak van der Waals forces. Sb2Te3 is a narrow-gap semiconductor with a band gap 0.21 eV; it is also a topological insulator, and thus exhibits thickness-dependent physical properties.
Crystallization of polymers is a process associated with partial alignment of their molecular chains. These chains fold together and form ordered regions called lamellae, which compose larger spheroidal structures named spherulites. Polymers can crystallize upon cooling from melting, mechanical stretching or solvent evaporation. Crystallization affects optical, mechanical, thermal and chemical properties of the polymer. The degree of crystallinity is estimated by different analytical methods and it typically ranges between 10 and 80%, with crystallized polymers often called "semi-crystalline". The properties of semi-crystalline polymers are determined not only by the degree of crystallinity, but also by the size and orientation of the molecular chains.

Crystalline silicon or (c-Si) Is the crystalline forms of silicon, either polycrystalline silicon, or monocrystalline silicon. Crystalline silicon is the dominant semiconducting material used in photovoltaic technology for the production of solar cells. These cells are assembled into solar panels as part of a photovoltaic system to generate solar power from sunlight.
Chalcogenide chemical vapour deposition is a proposed technology for depositing thin films of chalcogenides, i.e. materials derived from sulfides, selenides, and tellurides. Conventional CVD can be used to deposit films of most metals, many non-metallic elements as well as a large number of compounds including carbides, nitrides, oxides. CVD can be used to synthesize chalcogenide glasses.
Hoffman nucleation theory is a theory developed by John D. Hoffman and coworkers in the 1970s and 80s that attempts to describe the crystallization of a polymer in terms of the kinetics and thermodynamics of polymer surface nucleation. The theory introduces a model where a surface of completely crystalline polymer is created and introduces surface energy parameters to describe the process. Hoffman nucleation theory is more of a starting point for polymer crystallization theory and is better known for its fundamental roles in the Hoffman–Weeks lamellar thickening and Lauritzen–Hoffman growth theory.

Lithium aluminium germanium phosphate, typically known with the acronyms LAGP or LAGPO, is an inorganic ceramic solid material whose general formula is Li
1+xAl
xGe
2-x(PO
4)
3. LAGP belongs to the NASICON family of solid conductors and has been applied as a solid electrolyte in all-solid-state lithium-ion batteries. Typical values of ionic conductivity in LAGP at room temperature are in the range of 10–5 - 10–4 S/cm, even if the actual value of conductivity is strongly affected by stoichiometry, microstructure, and synthesis conditions. Compared to lithium aluminium titanium phosphate (LATP), which is another phosphate-based lithium solid conductor, the absence of titanium in LAGP improves its stability towards lithium metal. In addition, phosphate-based solid electrolytes have superior stability against moisture and oxygen compared to sulfide-based electrolytes like Li
10GeP
2S
12 (LGPS) and can be handled safely in air, thus simplifying the manufacture process. Since the best performances are encountered when the stoichiometric value of x is 0.5, the acronym LAGP usually indicates the particular composition of Li
1.5Al
0.5Ge
1.5(PO
4)
3, which is also the typically used material in battery applications.














