
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored.
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate BO3−3, metaborate BO−2, or tetraborate B4O2−7; or any salt of such anions, such as sodium metaborate, Na+[BO2]− and borax (Na+)2[B4O7]2−. The name also refers to esters of such anions, such as trimethyl borate B(OCH3)3 but they are alkoxides.
Pervaporation is a processing method for the separation of mixtures of liquids by partial vaporization through a non-porous or porous membrane.

Fused quartz, fused silica or quartz glass is a glass consisting of almost pure silica (silicon dioxide, SiO2) in amorphous (non-crystalline) form. This differs from all other commercial glasses, such as soda-lime glass, lead glass, or borosilicate glass, in which other ingredients are added which change the glasses' optical and physical properties, such as lowering the melt temperature, the spectral transmission range, or the mechanical strength. Fused quartz, therefore, has high working and melting temperatures, making it difficult to form and less desirable for most common applications, but is much stronger, more chemically resistant, and exhibits lower thermal expansion, making it more suitable for many specialized uses such as lighting and scientific applications.

In materials science, a refractory is a material that is resistant to decomposition by heat or chemical attack that retains its strength and rigidity at high temperatures. They are inorganic, non-metallic compounds that may be porous or non-porous, and their crystallinity varies widely: they may be crystalline, polycrystalline, amorphous, or composite. They are typically composed of oxides, carbides or nitrides of the following elements: silicon, aluminium, magnesium, calcium, boron, chromium and zirconium. Many refractories are ceramics, but some such as graphite are not, and some ceramics such as clay pottery are not considered refractory. Refractories are distinguished from the refractory metals, which are elemental metals and their alloys that have high melting temperatures.
In semiconductor manufacturing, a low-κ is a material with a small relative dielectric constant relative to silicon dioxide. Low-κ dielectric material implementation is one of several strategies used to allow continued scaling of microelectronic devices, colloquially referred to as extending Moore's law. In digital circuits, insulating dielectrics separate the conducting parts from one another. As components have scaled and transistors have gotten closer together, the insulating dielectrics have thinned to the point where charge build up and crosstalk adversely affect the performance of the device. Replacing the silicon dioxide with a low-κ dielectric of the same thickness reduces parasitic capacitance, enabling faster switching speeds and lower heat dissipation. In conversation such materials may be referred to as "low-k" rather than "low-κ" (low-kappa).

Borosilicate glass is a type of glass with silica and boron trioxide as the main glass-forming constituents. Borosilicate glasses are known for having very low coefficients of thermal expansion, making them more resistant to thermal shock than any other common glass. Such glass is subjected to less thermal stress and can withstand temperature differentials without fracturing of about 165 °C (300 °F). It is commonly used for the construction of reagent bottles and flasks, as well as lighting, electronics, and cookware.
An artificial membrane, or synthetic membrane, is a synthetically created membrane which is usually intended for separation purposes in laboratory or in industry. Synthetic membranes have been successfully used for small and large-scale industrial processes since the middle of the twentieth century. A wide variety of synthetic membranes is known. They can be produced from organic materials such as polymers and liquids, as well as inorganic materials. Most commercially utilized synthetic membranes in industry are made of polymeric structures. They can be classified based on their surface chemistry, bulk structure, morphology, and production method. The chemical and physical properties of synthetic membranes and separated particles as well as separation driving force define a particular membrane separation process. The most commonly used driving forces of a membrane process in industry are pressure and concentration gradient. The respective membrane process is therefore known as filtration. Synthetic membranes utilized in a separation process can be of different geometry and flow configurations. They can also be categorized based on their application and separation regime. The best known synthetic membrane separation processes include water purification, reverse osmosis, dehydrogenation of natural gas, removal of cell particles by microfiltration and ultrafiltration, removal of microorganisms from dairy products, and dialysis.
Vycor is the brand name of Corning's high-silica, high-temperature glass. It provides very high thermal shock resistance. Vycor is approximately 96% silica and 4% boron trioxide, but unlike pure fused silica, it can be readily manufactured in a variety of shapes.Vycor can be subject to prolonged usage at 900°C.

A molecular sieve is a material with pores of uniform size. These pore diameters are similar in size to small molecules, and thus large molecules cannot enter or be adsorbed, while smaller molecules can. As a mixture of molecules migrates through the stationary bed of porous, semi-solid substance referred to as a sieve, the components of the highest molecular weight leave the bed first, followed by successively smaller molecules. Some molecular sieves are used in size-exclusion chromatography, a separation technique that sorts molecules based on their size. Another important use is as a desiccant. Most of molecular sieves are aluminosilicate zeolites with Si/Al molar ratio less than 2, but there are also examples of activated charcoal and silica gel.
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers in solution into a colloidal solution (sol) that acts as the precursor for an integrated network of either discrete particles or network polymers. Typical precursors are metal alkoxides. Sol–gel process is used to produce ceramic nanoparticles.

Bioglass 45S5 or calcium sodium phosphosilicate, is a bioactive glass specifically composed of 45 wt% SiO2, 24.5 wt% CaO, 24.5 wt% Na2O, and 6.0 wt% P2O5. Typical applications of Bioglass 45S5 include: bone grafting biomaterials, repair of periodontal defects, cranial and maxillofacial repair, wound care, blood loss control, stimulation of vascular regeneration, and nerve repair.
Bioactive glasses are a group of surface reactive glass-ceramic biomaterials and include the original bioactive glass, Bioglass. The biocompatibility and bioactivity of these glasses has led them to be used as implant devices in the human body to repair and replace diseased or damaged bones. Most bioactive glasses are silicate-based glasses that are degradable in body fluids and can act as a vehicle for delivering ions beneficial for healing. Bioactive glass is differentiated from other synthetic bone grafting biomaterials, in that it is the only one with anti-infective and angiogenic properties.

Nanoporous materials consist of a regular organic or inorganic bulk phase in which a porous structure is present. Nanoporous materials exhibit pore diameters that are most appropriately quantified using units of nanometers. The diameter of pores in nanoporous materials is thus typically 100 nanometers or smaller. Pores may be open or closed, and pore connectivity and void fraction vary considerably, as with other porous materials. Open pores are pores that connect to the surface of the material whereas closed pores are pockets of void space within a bulk material. Open pores are useful for molecular separation techniques, adsorption, and catalysis studies. Closed pores are mainly used in thermal insulators and for structural applications.
Ceramic foam is a tough foam made from ceramics. Manufacturing techniques include impregnating open-cell polymer foams internally with ceramic slurry and then firing in a kiln, leaving only ceramic material. The foams may consist of several ceramic materials such as aluminium oxide, a common high-temperature ceramic, and gets insulating properties from the many tiny air-filled voids within the material.
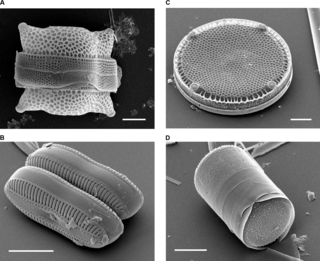
A frustule is the hard and porous cell wall or external layer of diatoms. The frustule is composed almost purely of silica, made from silicic acid, and is coated with a layer of organic substance, which was referred to in the early literature on diatoms as pectin, a fiber most commonly found in cell walls of plants. This layer is actually composed of several types of polysaccharides.
Dealkalization is a process of surface modification applicable to glasses containing alkali ions, wherein a thin surface layer is created that has a lower concentration of alkali ions than is present in the underlying, bulk glass. This change in surface composition commonly alters the observed properties of the surface, most notably enhancing corrosion resistance.

Zeolitic imidazolate frameworks (ZIFs) are a class of metal-organic frameworks (MOFs) that are topologically isomorphic with zeolites. ZIFs are composed of tetrahedrally-coordinated transition metal ions connected by imidazolate linkers. Since the metal-imidazole-metal angle is similar to the 145° Si-O-Si angle in zeolites, ZIFs have zeolite-like topologies. As of 2010, 105 ZIF topologies have been reported in the literature. Due to their robust porosity, resistance to thermal changes, and chemical stability, ZIFs are being investigated for applications such as carbon dioxide capture.

Spin column-based nucleic acid purification is a solid phase extraction method to quickly purify nucleic acids. This method relies on the fact that nucleic acid will bind to the solid phase of silica under certain conditions.
Precipitated silica is an amorphous form of silica (silicon dioxide, SiO2); it is a white, powdery material. Precipitated silica is produced by precipitation from a solution containing silicate salts.












