Iontophoresis is a process of transdermal drug delivery by use of a voltage gradient on the skin. Molecules are transported across the stratum corneum by electrophoresis and electroosmosis and the electric field can also increase the permeability of the skin. These phenomena, directly and indirectly, constitute active transport of matter due to an applied electric current. The transport is measured in units of chemical flux, commonly μmol/(cm2*hour). Iontophoresis has experimental, therapeutic and diagnostic applications.

Dendrimers are highly ordered, branched polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The word dendron is also encountered frequently. A dendron usually contains a single chemically addressable group called the focal point or core. The difference between dendrons and dendrimers is illustrated in the top figure, but the terms are typically encountered interchangeably.

Robert Samuel Langer Jr. FREng is an American chemical engineer, scientist, entrepreneur, inventor and one of the twelve Institute Professors at the Massachusetts Institute of Technology.
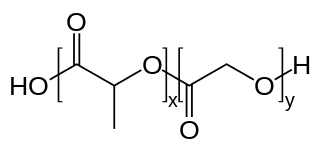
PLGA, PLG, or poly(lactic-co-glycolic acid) is a copolymer which is used in a host of Food and Drug Administration (FDA) approved therapeutic devices, owing to its biodegradability and biocompatibility. PLGA is synthesized by means of ring-opening co-polymerization of two different monomers, the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid. Polymers can be synthesized as either random or block copolymers thereby imparting additional polymer properties. Common catalysts used in the preparation of this polymer include tin(II) 2-ethylhexanoate, tin(II) alkoxides, or aluminum isopropoxide. During polymerization, successive monomeric units are linked together in PLGA by ester linkages, thus yielding a linear, aliphatic polyester as a product.

Drug delivery refers to approaches, formulations, manufacturing techniques, storage systems, and technologies involved in transporting a pharmaceutical compound to its target site to achieve a desired therapeutic effect. Principles related to drug preparation, route of administration, site-specific targeting, metabolism, and toxicity are used to optimize efficacy and safety, and to improve patient convenience and compliance. Drug delivery is aimed at altering a drug's pharmacokinetics and specificity by formulating it with different excipients, drug carriers, and medical devices. There is additional emphasis on increasing the bioavailability and duration of action of a drug to improve therapeutic outcomes. Some research has also been focused on improving safety for the person administering the medication. For example, several types of microneedle patches have been developed for administering vaccines and other medications to reduce the risk of needlestick injury.
Targeted drug delivery, sometimes called smart drug delivery, is a method of delivering medication to a patient in a manner that increases the concentration of the medication in some parts of the body relative to others. This means of delivery is largely founded on nanomedicine, which plans to employ nanoparticle-mediated drug delivery in order to combat the downfalls of conventional drug delivery. These nanoparticles would be loaded with drugs and targeted to specific parts of the body where there is solely diseased tissue, thereby avoiding interaction with healthy tissue. The goal of a targeted drug delivery system is to prolong, localize, target and have a protected drug interaction with the diseased tissue. The conventional drug delivery system is the absorption of the drug across a biological membrane, whereas the targeted release system releases the drug in a dosage form. The advantages to the targeted release system is the reduction in the frequency of the dosages taken by the patient, having a more uniform effect of the drug, reduction of drug side-effects, and reduced fluctuation in circulating drug levels. The disadvantage of the system is high cost, which makes productivity more difficult, and the reduced ability to adjust the dosages.
Sonophoresis is a method that utilizes ultrasound to enhance the delivery of topical medications through the stratum corneum, to the epidermis and dermis. Sonophoresis allows for the enhancement of the permeability of the skin along with other modalities, such as iontophoresis, to deliver drugs with lesser side effects. Currently, sonophoresis is used widely in transdermal drug delivery, but has potential applications in other sectors of drug delivery, such as the delivery of drugs to the eye and brain.

Phonophoresis, also known as sonophoresis, is the method of using ultrasound waves to increase skin permeability in order to improve the effectiveness of transdermal drug delivery. This method intersects drug delivery and ultrasound therapy. Phonophoresis is able to achieve specific and efficient delivery of drugs that are delivered through the skin and ensure that the drug reaches the targeted area in the tissue environment. By assisting transdermal drug delivery, phonophoresis can help patients experience painless, minimal risk, and effective treatment.
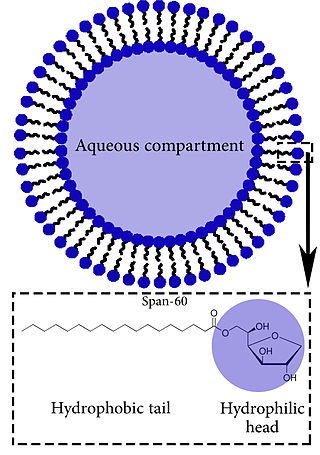
Niosomes are non-ionic surfactant-based vesicles that include non-ionic surfactant and cholesterol as an excipient, utilized for drug delivery to specific sites to achieve desired therapeutic effects. Structurally, niosomes are similar to liposomes, as they both consist of a lipid bilayer. However, niosomes are more stable than liposomes during formation processes and storage. They can trap hydrophilic and lipophilic drugs, either in an aqueous compartment or in a vesicular membrane compartment composed of lipid material.
A nanogel is a polymer-based, crosslinked hydrogel particle on the sub-micron scale. These complex networks of polymers present a unique opportunity in the field of drug delivery at the intersection of nanoparticles and hydrogel synthesis. Nanogels can be natural, synthetic, or a combination of the two and have a high degree of tunability in terms of their size, shape, surface functionalization, and degradation mechanisms. Given these inherent characteristics in addition to their biocompatibility and capacity to encapsulate small drugs and molecules, nanogels are a promising strategy to treat disease and dysfunction by serving as delivery vehicles capable of navigating across challenging physiological barriers within the body.
Microbubbles (MBs) are bubbles smaller than one hundredth of a millimetre in diameter, but larger than one micrometre. They have widespread application in industry, medicine, life science, and food technology. The composition of the bubble shell and filling material determine important design features such as buoyancy, crush strength, thermal conductivity, and acoustic properties.

Samir Mitragotri is an Indian American professor at Harvard University, an inventor, an entrepreneur, and a researcher in the fields of drug delivery and biomaterials. He is currently the Hiller Professor of Bioengineering and Hansjörg Wyss Professor of Biologically Inspired Engineering at Harvard John A. Paulson School of Engineering and Applied Sciences and the Wyss Institute for Biologically Inspired Engineering. Prior to 2017, he was the Duncan and Suzanne Mellichamp Chair Professor at University of California, Santa Barbara.

Microneedles or Microneedle patches or Microarray patches are micron-scaled medical devices used to administer vaccines, drugs, and other therapeutic agents. While microneedles were initially explored for transdermal drug delivery applications, their use has been extended for the intraocular, vaginal, transungual, cardiac, vascular, gastrointestinal, and intracochlear delivery of drugs. Microneedles are constructed through various methods, usually involving photolithographic processes or micromolding. These methods involve etching microscopic structure into resin or silicon in order to cast microneedles. Microneedles are made from a variety of material ranging from silicon, titanium, stainless steel, and polymers. Some microneedles are made of a drug to be delivered to the body but are shaped into a needle so they will penetrate the skin. The microneedles range in size, shape, and function but are all used as an alternative to other delivery methods like the conventional hypodermic needle or other injection apparatus.
Hamid Ghandehari is an Iranian-American drug delivery research scientist, and a professor in the Departments of Pharmaceutics and Pharmaceutical Chemistry and Biomedical Engineering at the University of Utah. His research is focused in recombinant polymers for drug and gene delivery, nanotoxicology of dendritic and inorganic constructs, water-soluble polymers for targeted delivery and poly(amidoamine) dendrimers for oral delivery.

Mark Robert Prausnitz is an American chemical engineer, currently Regents’ Professor and J. Erskine Love, Jr. Chair in Chemical & Biomolecular Engineering at the Georgia Institute of Technology, He also serves as adjunct professor of biomedical engineering at Emory University and Adjunct Professor of Chemical & Biomolecular Engineering at the Korea Advanced Institute of Science and Technology. He is known for pioneering microneedle technology for minimally invasive drug and vaccine administration, which has found applications in transdermal, ocular, oral, and sustained release delivery systems.
Pullulan bioconjugates are systems that use pullulan as a scaffold to attach biological materials to, such as drugs. These systems can be used to enhance the delivery of drugs to specific environments or the mechanism of delivery. These systems can be used in order to deliver drugs in response to stimuli, create a more controlled and sustained release, and provide a more targeted delivery of certain drugs.
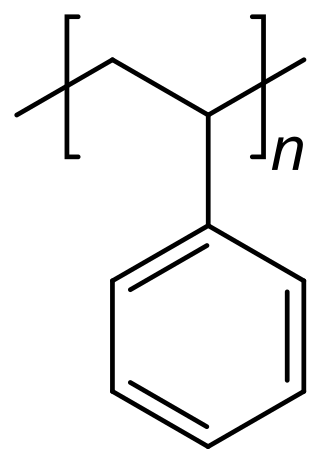
Polystyrene is a synthetic hydrocarbon polymer that is widely adaptive and can be used for a variety of purposes in drug delivery. These methods include polystyrene microspheres, nanoparticles, and solid foams. In the biomedical engineering field, these methods assist researchers in drug delivery, diagnostics, and imaging strategies.

Alexander Viktorovich Kabanov, is a Russian and American chemist, an educator, an entrepreneur, and a researcher in the fields of drug delivery and nanomedicine.
Ultrasound-triggered drug delivery using stimuli-responsive hydrogels refers to the process of using ultrasound energy for inducing drug release from hydrogels that are sensitive to acoustic stimuli. This method of approach is one of many stimuli-responsive drug delivery-based systems that has gained traction in recent years due to its demonstration of localization and specificity of disease treatment. Although recent developments in this field highlight its potential in treating certain diseases such as COVID-19, there remain many major challenges that need to be addressed and overcome before more related biomedical applications are clinically translated into standard of care.
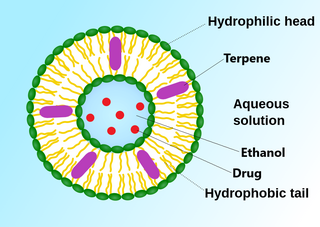
An invasome are a type of artificial vesicle nanocarrier that transport substances through the skin, the most superficial biological barrier. Vesicles are small particles surrounded by a lipid layer that can carry substances into and out of the cell. Artificial vesicles can be engineered to deliver drugs within the cell, with specific applications within transdermal drug delivery. However, the skin proves to be a barrier to effective penetration and delivery of drug therapies. Thus, invasomes are a new generation of vesicle with added structural components to assist with skin penetration.













