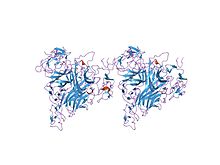
Tumor necrosis factor (TNF), formerly known as TNF-α, is an inflammatory protein and a principal mediator of the innate immune response. TNF is produced primarily by macrophages in response to antigens, and activates inflammatory pathways through its two receptors, TNFR1 and TNFR2. It is a member of the tumor necrosis factor superfamily, a family of type II transmembrane proteins that function as cytokines. Excess production of TNF plays a critical role in the pathology of several inflammatory diseases, and anti-TNF therapies are often employed to treat these diseases.

Fas ligand is a type-II transmembrane protein expressed on various types of cells, including cytotoxic T lymphocytes, monocytes, neutrophils, breast epithelial cells, vascular endothelial cells and natural killer (NK) cells. It binds with its receptor, called FAS receptor and plays a crucial role in the regulation of the immune system and in induction of apoptosis, a programmed cell death.

In the field of cell biology, TNF-related apoptosis-inducing ligand (TRAIL), is a protein functioning as a ligand that induces the process of cell death called apoptosis.

The tumor necrosis factor receptor superfamily (TNFRSF) is a protein superfamily of cytokine receptors characterized by the ability to bind tumor necrosis factors (TNFs) via an extracellular cysteine-rich domain. With the exception of nerve growth factor (NGF), all TNFs are homologous to the archetypal TNF-alpha. In their active form, the majority of TNF receptors form trimeric complexes in the plasma membrane. Accordingly, most TNF receptors contain transmembrane domains (TMDs), although some can be cleaved into soluble forms, and some lack a TMD entirely. In addition, most TNF receptors require specific adaptor protein such as TRADD, TRAF, RIP and FADD for downstream signalling. TNF receptors are primarily involved in apoptosis and inflammation, but they can also take part in other signal transduction pathways, such as proliferation, survival, and differentiation. TNF receptors are expressed in a wide variety of tissues in mammals, especially in leukocytes.
Lymphotoxin is a member of the tumor necrosis factor (TNF) superfamily of cytokines, whose members are responsible for regulating the growth and function of lymphocytes and are expressed by a wide variety of cells in the body.

Tumor necrosis factor receptor 1 (TNFR1), also known as tumor necrosis factor receptor superfamily member 1A (TNFRSF1A) and CD120a, is a ubiquitous membrane receptor that binds tumor necrosis factor-alpha (TNFα).

TNF receptor-associated factor 1 is a protein that in humans is encoded by the TRAF1 gene.

TNF receptor-associated factor (TRAF3) is a protein that in humans is encoded by the TRAF3 gene.

A proliferation-inducing ligand (APRIL), also known as tumor necrosis factor ligand superfamily member 13 (TNFSF13), is a protein of the TNF superfamily recognized by the cell surface receptor TACI. It is encoded by the TNFSF13 gene.

LIGHT, also known as tumor necrosis factor superfamily member 14 (TNFSF14), is a secreted protein of the TNF superfamily. It is recognized by herpesvirus entry mediator (HVEM), as well as decoy receptor 3.

Herpesvirus entry mediator (HVEM), also known as tumor necrosis factor receptor superfamily member 14 (TNFRSF14), is a human cell surface receptor of the TNF-receptor superfamily encoded by the TNFRSF14 gene.

Transmembrane activator and CAML interactor (TACI), also known as tumor necrosis factor receptor superfamily member 13B (TNFRSF13B) is a protein that in humans is encoded by the TNFRSF13B gene.

Decoy receptor 2 (DCR2), also known as TRAIL receptor 4 (TRAILR4) and tumor necrosis factor receptor superfamily member 10D (TNFRSF10D), is a human cell surface receptor of the TNF-receptor superfamily.

B-cell maturation antigen, also known as tumor necrosis factor receptor superfamily member 17 (TNFRSF17), is a protein that in humans is encoded by the TNFRSF17 gene.

Tumor necrosis factor receptor superfamily member 18 (TNFRSF18), also known as glucocorticoid-induced TNFR-related protein (GITR) or CD357. GITR is encoded and tnfrsf18 gene at chromosome 4 in mice. GITR is type I transmembrane protein and is described in 4 different isoforms. GITR human orthologue, also called activation-inducible TNFR family receptor (AITR), is encoded by the TNFRSF18 gene at chromosome 1.

Death receptor 6 (DR6), also known as tumor necrosis factor receptor superfamily member 21 (TNFRSF21), is a cell surface receptor of the tumor necrosis factor receptor superfamily which activates the JNK and NF-κB pathways. It is mostly expressed in the thymus, spleen and white blood cells. The Gene for DR6 is 78,450 bases long and is found on the 6th chromosome. This is transcribed into a 655 amino acid chain weighing 71.8 kDa. Post transcriptional modifications of this protein include glycosylation on the asparagines at the 82, 141, 252, 257, 278, and 289 amino acid locations.

Tumor necrosis factor (ligand) superfamily, member 12-member 13, also known as TNFSF12-TNFSF13, is a human gene.

Tumor necrosis factor receptor 2 (TNFR2), also known as tumor necrosis factor receptor superfamily member 1B (TNFRSF1B) and CD120b, is one of two membrane receptors that binds tumor necrosis factor-alpha (TNFα). Like its counterpart, tumor necrosis factor receptor 1 (TNFR1), the extracellular region of TNFR2 consists of four cysteine-rich domains which allow for binding to TNFα. TNFR1 and TNFR2 possess different functions when bound to TNFα due to differences in their intracellular structures, such as TNFR2 lacking a death domain (DD).

Tumor necrosis factor receptor superfamily member 19L is a protein that in humans is encoded by the RELT gene.
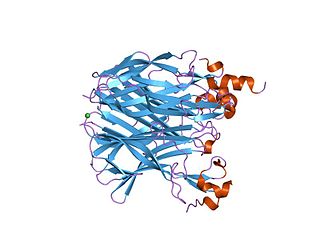
In molecular biology, TACI-CRD2 represents the second cysteine-rich protein domain found in the TACI family of proteins. Members of this family are predominantly found in tumour necrosis factor receptor superfamily, member 13b (TACI), and are required for binding to the ligands APRIL and BAFF. TACI-CRD2 stands for Transmembrane Activator and CAML Interactor- Cysteine Rich Domain 2.