
Photosynthesis is a biological process used by many cellular organisms to convert light energy into chemical energy, which is stored in organic compounds that can later be metabolized through cellular respiration to fuel the organism's activities. The term usually refers to oxygenic photosynthesis, where oxygen is produced as a byproduct, and some of the chemical energy produced is stored in carbohydrate molecules such as sugars, starch and cellulose, which are synthesized from endergonic reaction of carbon dioxide with water. Most plants, algae and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis is largely responsible for producing and maintaining the oxygen content of the Earth's atmosphere, and supplies most of the biological energy necessary for complex life on Earth.

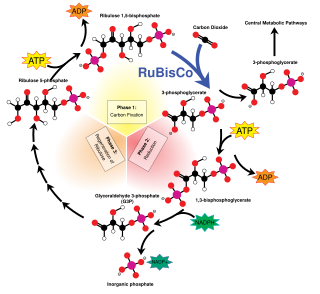
Ribulose-1,5-bisphosphate carboxylase/oxygenase, commonly known by the abbreviations RuBisCo, rubisco, RuBPCase, or RuBPco, is an enzyme involved in light-independent part of photosynthesis, including the carbon fixation by which atmospheric carbon dioxide is converted by plants and other photosynthetic organisms to energy-rich molecules such as glucose. It emerged approximately four billion years ago in primordial metabolism prior to the presence of oxygen on earth. It is probably the most abundant enzyme on Earth. In chemical terms, it catalyzes the carboxylation of ribulose-1,5-bisphosphate.

C4 carbon fixation or the Hatch–Slack pathway is one of three known photosynthetic processes of carbon fixation in plants. It owes the names to the 1960s discovery by Marshall Davidson Hatch and Charles Roger Slack that some plants, when supplied with 14CO2, incorporate the 14C label into four-carbon molecules first.

Photorespiration (also known as the oxidative photosynthetic carbon cycle or C2 cycle) refers to a process in plant metabolism where the enzyme RuBisCO oxygenates RuBP, wasting some of the energy produced by photosynthesis. The desired reaction is the addition of carbon dioxide to RuBP (carboxylation), a key step in the Calvin–Benson cycle, but approximately 25% of reactions by RuBisCO instead add oxygen to RuBP (oxygenation), creating a product that cannot be used within the Calvin–Benson cycle. This process lowers the efficiency of photosynthesis, potentially lowering photosynthetic output by 25% in C3 plants. Photorespiration involves a complex network of enzyme reactions that exchange metabolites between chloroplasts, leaf peroxisomes and mitochondria.

Ribulose 1,5-bisphosphate (RuBP) is an organic substance that is involved in photosynthesis, notably as the principal CO2 acceptor in plants. It is a colourless anion, a double phosphate ester of the ketopentose called ribulose. Salts of RuBP can be isolated, but its crucial biological function happens in solution. RuBP occurs not only in plants but in all domains of life, including Archaea, Bacteria, and Eukarya.
C3 carbon fixation is the most common of three metabolic pathways for carbon fixation in photosynthesis, the other two being C4 and CAM. This process converts carbon dioxide and ribulose bisphosphate (RuBP, a 5-carbon sugar) into two molecules of 3-phosphoglycerate through the following reaction:
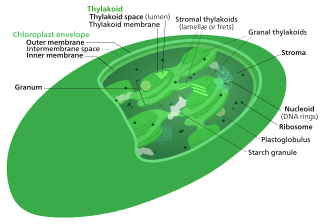
The Calvin cycle,light-independent reactions, bio synthetic phase,dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into glucose. The Calvin cycle is present in all photosynthetic eukaryotes and also many photosynthetic bacteria. In plants, these reactions occur in the stroma, the fluid-filled region of a chloroplast outside the thylakoid membranes. These reactions take the products of light-dependent reactions and perform further chemical processes on them. The Calvin cycle uses the chemical energy of ATP and reducing power of NADPH from the light dependent reactions to produce sugars for the plant to use. These substrates are used in a series of reduction-oxidation reactions to produce sugars in a step-wise process; there is no direct reaction that converts several molecules of CO2 to a sugar. There are three phases to the light-independent reactions, collectively called the Calvin cycle: carboxylation, reduction reactions, and ribulose 1,5-bisphosphate (RuBP) regeneration.
Methylotrophs are a diverse group of microorganisms that can use reduced one-carbon compounds, such as methanol or methane, as the carbon source for their growth; and multi-carbon compounds that contain no carbon-carbon bonds, such as dimethyl ether and dimethylamine. This group of microorganisms also includes those capable of assimilating reduced one-carbon compounds by way of carbon dioxide using the ribulose bisphosphate pathway. These organisms should not be confused with methanogens which on the contrary produce methane as a by-product from various one-carbon compounds such as carbon dioxide. Some methylotrophs can degrade the greenhouse gas methane, and in this case they are called methanotrophs. The abundance, purity, and low price of methanol compared to commonly used sugars make methylotrophs competent organisms for production of amino acids, vitamins, recombinant proteins, single-cell proteins, co-enzymes and cytochromes.
The light compensation point (Ic) is the light intensity on the light curve where the rate of photosynthesis exactly matches the rate of cellular respiration. At this point, the uptake of CO2 through photosynthetic pathways is equal to the respiratory release of carbon dioxide, and the uptake of O2 by respiration is equal to the photosynthetic release of oxygen. The concept of compensation points in general may be applied to other photosynthetic variables, the most important being that of CO2 concentration – CO2 compensation point (Γ).Interval of time in day time when light intensity is low due to which net gaseous exchange is zero is called as compensation point.
The photosynthetic efficiency is the fraction of light energy converted into chemical energy during photosynthesis in green plants and algae. Photosynthesis can be described by the simplified chemical reaction
Phosphoenolpyruvate carboxylase (also known as PEP carboxylase, PEPCase, or PEPC; EC 4.1.1.31, PDB ID: 3ZGE) is an enzyme in the family of carboxy-lyases found in plants and some bacteria that catalyzes the addition of bicarbonate (HCO3−) to phosphoenolpyruvate (PEP) to form the four-carbon compound oxaloacetate and inorganic phosphate:
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylation, especially when applied to the reaction of carbanionic reagents with CO2. More generally, carbonation usually describes the production of carbonates.
In enzymology, a [ribulose-bisphosphate carboxylase]-lysine N-methyltransferase (EC 2.1.1.127) is an enzyme that catalyzes the chemical reaction
Phosphoribulokinase (PRK) (EC 2.7.1.19) is an essential photosynthetic enzyme that catalyzes the ATP-dependent phosphorylation of ribulose 5-phosphate (RuP) into ribulose 1,5-bisphosphate (RuBP), both intermediates in the Calvin Cycle. Its main function is to regenerate RuBP, which is the initial substrate and CO2-acceptor molecule of the Calvin Cycle. PRK belongs to the family of transferase enzymes, specifically those transferring phosphorus-containing groups (phosphotransferases) to an alcohol group acceptor. Along with ribulose 1,5-bisphosphate carboxylase/oxygenase (RuBisCo), phosphoribulokinase is unique to the Calvin Cycle. Therefore, PRK activity often determines the metabolic rate in organisms for which carbon fixation is key to survival. Much initial work on PRK was done with spinach leaf extracts in the 1950s; subsequent studies of PRK in other photosynthetic prokaryotic and eukaryotic organisms have followed. The possibility that PRK might exist was first recognized by Weissbach et al. in 1954; for example, the group noted that carbon dioxide fixation in crude spinach extracts was enhanced by the addition of ATP. The first purification of PRK was conducted by Hurwitz and colleagues in 1956.
ATP + Mg2+ - D-ribulose 5-phosphate ADP + D-ribulose 1,5-bisphosphate
Photosynthetic capacity (Amax) is a measure of the maximum rate at which leaves are able to fix carbon during photosynthesis. It is typically measured as the amount of carbon dioxide that is fixed per metre squared per second, for example as μmol m−2 sec−1.
[Fructose-bisphosphate aldolase]-lysine N-methyltransferase (EC 2.1.1.259) is an enzyme that catalyses the following chemical reaction

Position-specific isotope analysis, also called site-specific isotope analysis, is a branch of isotope analysis aimed at determining the isotopic composition of a particular atom position in a molecule. Isotopes are elemental variants with different numbers of neutrons in their nuclei, thereby having different atomic masses. Isotopes are found in varying natural abundances depending on the element; their abundances in specific compounds can vary from random distributions due to environmental conditions that act on the mass variations differently. These differences in abundances are called "fractionations," which are characterized via stable isotope analysis.
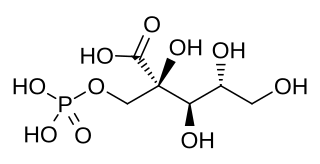
2-Carboxy-D-arabitinol 1-phosphate is a molecule produced in plants that inhibits RuBisCO, a key enzyme in the Calvin cycle and carbon fixation. In dark conditions, this molecule binds to RuBisCO, preventing it from participating in chemical reactions. As the amount of light present increases, CA1P levels decrease, freeing RuBisCO's reactive ends, allowing more of the molecules to participate in chemical reactions. It can be broken down by the enzyme 2-carboxy-D-arabinitol-1-phosphatase into 2-carboxy-D-arabinitol.

Photosynthesis converts carbon dioxide to carbohydrates via several metabolic pathways that provide energy to an organism and preferentially react with certain stable isotopes of carbon. The selective enrichment of one stable isotope over another creates distinct isotopic fractionations that can be measured and correlated among oxygenic phototrophs. The degree of carbon isotope fractionation is influenced by several factors, including the metabolism, anatomy, growth rate, and environmental conditions of the organism. Understanding these variations in carbon fractionation across species is useful for biogeochemical studies, including the reconstruction of paleoecology, plant evolution, and the characterization of food chains.

2-Phosphoglycolate (chemical formula C2H2O6P3-; also known as phosphoglycolate, 2-PG, or PG) is a natural metabolic product of the oxygenase reaction mediated by the enzyme ribulose 1,5-bisphosphate carboxylase (RuBisCo).


















