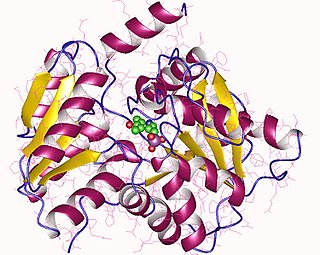
| L-2-amino-4-chloropent-4-enoate dehydrochlorinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 4.5.1.4 | ||||||||
| CAS no. | 113066-37-8 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
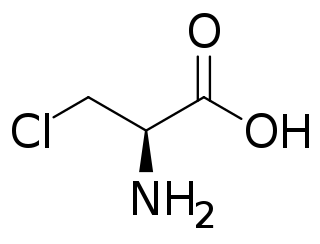
The enzyme L-2-amino-4-chloropent-4-enoate dehydrochlorinase (EC 4.5.1.4) catalyzes the reaction
- L-2-amino-4-chloropent-4-enoate + H2O 2-oxopent-4-enoate + chloride + NH3
This enzyme belongs to the family of lyases, specifically the class of carbon-halide lyases. The systematic name of this enzyme class is L-2-amino-4-chloropent-4-enoate chloride-lyase (adding water; deaminating; 2-oxopent-4-enoate-forming). Other names in common use include L-2-amino-4-chloro-4-pentenoate dehalogenase, and L-2-amino-4-chloropent-4-enoate chloride-lyase (deaminating).