
Carotenoids, also called tetraterpenoids, are yellow, orange, and red organic pigments that are produced by plants and algae, as well as several bacteria, and fungi. Carotenoids give the characteristic color to pumpkins, carrots, parsnips, corn, tomatoes, canaries, flamingos, salmon, lobster, shrimp, and daffodils. Carotenoids can be produced from fats and other basic organic metabolic building blocks by all these organisms. The only land dwelling arthropods known to produce carotenoids are aphids, and spider mites, which acquired the ability and genes from fungi. It is also produced by endosymbiotic bacteria in whiteflies. Carotenoids from the diet are stored in the fatty tissues of animals, and exclusively carnivorous animals obtain the compounds from animal fat. In the human diet, absorption of carotenoids is improved when consumed with fat in a meal. Cooking carotenoid-containing vegetables in oil and shredding the vegetable both increase carotenoid bioavailability.

Ergoline is a chemical compound whose structural skeleton is contained in a variety of alkaloids, referred to as ergoline derivatives or ergoline alkaloids. Ergoline alkaloids, one being ergine, were initially characterized in ergot. Some of these are implicated in the condition ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives.

Lindera benzoin is a shrub in the laurel family, native to eastern North America, ranging from Maine and New York to Ontario in the north, and to Kansas, Texas, and northern Florida in the center and south. Within its native range it is a relatively common plant where it grows in the understory in moist, rich woods, especially those with exposed limestone.

Dioscorea communis or Tamus communis is a species of flowering plant in the yam family Dioscoreaceae and is commonly known as black bryony, lady's-seal or black bindweed.

Lindera is a genus of about 80–100 species of flowering plants in the family Lauraceae, mostly native to eastern Asia but with three species in eastern North America. The species are shrubs and small trees; common names include spicewood, spicebush, and Benjamin bush.

Daidzein is a naturally occurring compound found exclusively in soybeans and other legumes and structurally belongs to a class of compounds known as isoflavones. Daidzein and other isoflavones are produced in plants through the phenylpropanoid pathway of secondary metabolism and are used as signal carriers, and defense responses to pathogenic attacks. In humans, recent research has shown the viability of using daidzein in medicine for menopausal relief, osteoporosis, blood cholesterol, and lowering the risk of some hormone-related cancers, and heart disease.

In molecular biology, hydroxymethylglutaryl-CoA synthase or HMG-CoA synthase EC 2.3.3.10 is an enzyme which catalyzes the reaction in which acetyl-CoA condenses with acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). This reaction comprises the second step in the mevalonate-dependent isoprenoid biosynthesis pathway. HMG-CoA is an intermediate in both cholesterol synthesis and ketogenesis. This reaction is overactivated in patients with diabetes mellitus type 1 if left untreated, due to prolonged insulin deficiency and the exhaustion of substrates for gluconeogenesis and the TCA cycle, notably oxaloacetate. This results in shunting of excess acetyl-CoA into the ketone synthesis pathway via HMG-CoA, leading to the development of diabetic ketoacidosis.

Aesculetin is a derivative of coumarin. It is a natural lactone that derives from the intramolecular cyclization of a cinnamic acid derivative.
Malaccensis may refer to:

Dihydrochalcone (DHC) is the organic compound with the formula C6H5C(O)(CH2)2C6H5. It is the reduced derivative of chalcone (C6H5C(O)(CH)2C6H5). It is white solid that is soluble in many organic solvents. Dihydrochalcone per se is often minor significance, but some derivatives occur in nature and have attracted attention as drugs.

Lindera aggregata is a plant species belonging to the genus Lindera.

Reticuline is a chemical compound found in a variety of plants including Lindera aggregata, Annona squamosa, and Ocotea fasciculata. It is based on the benzylisoquinoline structure.
L. malaccensis may refer to:
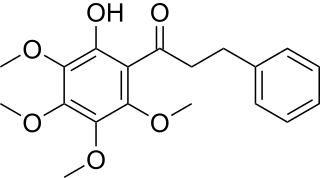
Dihydrokanakugiol is a dihydrochalcone isolated from Lindera lucida.

Alnetin is a flavone isolated from Lindera lucida.
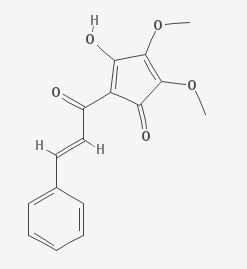
Linderone is a bio-active isolate of Lindera lucida.

Methyllinderone is a bio-active isolate of Lindera lucida.

Lindera subcoriacea, the bog spicebush, is a plant species native to the southeastern United States from Louisiana to Virginia. It is a shrub that can reach up to 4 m in height. Leaves are broadly elliptic, up to 8 cm long, and faintly aromatic when young. Flowers are yellow. Fruits are ellipsoid, deep red, about 10 mm long. It grows in acidic freshwater swamp forests in the Coastal Plain and Piedmont regions. In the northern portion of its range in the Carolinas and Virginia, L. subcoriacea is found only in the specialized stream pocosin habitat, while in the southern portion from Georgia onwards, it is found only in the wettest portions of the sphagnum bog habitat. As it is specialized and restricted to both of these very rare and unique ecosystems, L. subcoriacea is endangered due to habitat destruction and fire suppression. Only around 100 sites are known across this species' range to still sustain it, and most of these have only 1-5 genetically distinct individuals.

Homoisoflavonoids (3-benzylidenechroman-4-ones) are a type of phenolic compounds occurring naturally in plants.
Ledebouria floribunda is a species of flowering plant in the Asparagaceae family. It is found in Africa.
















