
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. These surrounded solute particles then move away from the solid solute and out into the solution. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when one of the solvents is water.

A solvent is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules, and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell.

In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.

Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent.

Extractive distillation is defined as distillation in the presence of a miscible, high-boiling, relatively non-volatile component, the solvent, that forms no azeotrope with the other components in the mixture. The method is used for mixtures having a low value of relative volatility, nearing unity. Such mixtures cannot be separated by simple distillation, because the volatility of the two components in the mixture is nearly the same, causing them to evaporate at nearly the same temperature at a similar rate, making normal distillation impractical.

Bromoform is an organic compound with the chemical formula CHBr3. It is a colorless liquid at room temperature, with a high refractive index and a very high density. Its sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. It is a brominated organic solvent. Currently its main use is as a laboratory reagent. It is very slightly soluble in water and is miscible with alcohol, benzene, chloroform, ether, petroleum ether, acetone and oils.
In chemistry, solvolysis is a type of nucleophilic substitution (SN1/SN2) or elimination where the nucleophile is a solvent molecule. Characteristic of SN1 reactions, solvolysis of a chiral reactant affords the racemate. Sometimes however, the stereochemical course is complicated by intimate ion pairs, whereby the leaving anion remains close to the carbocation, effectively shielding it from an attack by the nucleophile. Particularly fast reactions can occur by neighbour group participation, with nonclassical ions as intermediates or transition states.
1,1-Dichloroethane is a chlorinated hydrocarbon. It is a colorless oily liquid with a chloroform-like odor. It is not easily soluble in water, but miscible with most organic solvents.

Thiodiglycol, or bis(2-hydroxyethyl)sulfide (also known as 2,2-thiodiethanol or TDE), is the organosulfur compound with the formula S(CH2CH2OH)2. It is miscible with water and polar organic solvents. It is a colorless liquid. It is structurally similar to diethylene glycol.
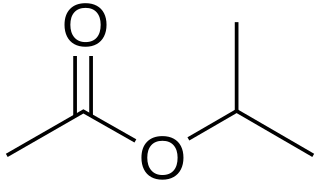
Isopropyl acetate is an ester, an organic compound which is the product of esterification of acetic acid and isopropanol. It is a clear, colorless liquid with a characteristic fruity odor.

2-Methoxyethanol, or methyl cellosolve, is an organic compound with formula C
3H
8O
2 that is used mainly as a solvent. It is a clear, colorless liquid with an ether-like odor. It is in a class of solvents known as glycol ethers which are notable for their ability to dissolve a variety of different types of chemical compounds and for their miscibility with water and other solvents. It can be formed by the nucleophilic attack of methanol on protonated ethylene oxide followed by proton transfer:

Ethyl pentanoate, also commonly known as ethyl valerate, is an organic compound used in flavors. It is an ester with the molecular formula C7H14O2. This colorless liquid is poorly soluble in water but miscible with organic solvents.
Crotyl alcohol, or crotonyl alcohol, is an unsaturated alcohol. It is a colourless liquid that is moderately soluble in water and miscible with most organic solvents. Two isomers of this alcohol exist, cis and trans.
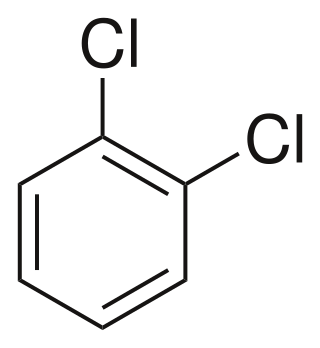
1,2-Dichlorobenzene, or orthodichlorobenzene (ODCB), is an aryl chloride and isomer of dichlorobenzene with the formula C6H4Cl2. This colourless liquid is poorly soluble in water but miscible with most organic solvents. It is a derivative of benzene, consisting of two adjacent chlorine atoms.

In fire protection, an accelerant is any substance or mixture that accelerates or speeds the development and escalation of fire. Accelerants are often used to commit arson, and some accelerants may cause an explosion. Some fire investigators use the term "accelerant" to mean any substance that initiates and promotes a fire without implying intent or malice. In Arson investigation, the significance of accelerant is to detect the presence of a such substance in order to proved that the fire is classified as an arson.

Miscibility is the property of two substances to mix in all proportions, forming a homogeneous mixture. The term is most often applied to liquids but also applies to solids and gases. An example in liquids is the miscibility of water and ethanol as they mix in all proportions.

The lower critical solution temperature (LCST) or lower consolute temperature is the critical temperature below which the components of a mixture are miscible in all proportions. The word lower indicates that the LCST is a lower bound to a temperature interval of partial miscibility, or miscibility for certain compositions only.
Isopropyl alcohol is a colorless, flammable organic compound with a pungent alcoholic odor.
A polyhalogenated compound (PHC) is any compound with multiple substitutions of halogens. They are of particular interest and importance because they bioaccumulate in humans, and comprise a superset of which has many toxic and carcinogenic industrial chemicals as members. PBDEs, PCBs, dioxins (PCDDs) and PFCs are all polyhalogenated compounds. They are generally non-miscible in organic solvents or water, but miscible in some hydrocarbons from which they often derive.

A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.