
Phenol is an aromatic organic compound with the molecular formula C6H5OH. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group bonded to a hydroxy group. Mildly acidic, it requires careful handling because it can cause chemical burns.

Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called epoxy. The IUPAC name for an epoxide group is an oxirane.

Dichloromethane is an organochlorine compound with the formula CH2Cl2. This colorless, volatile liquid with a chloroform-like, sweet odor is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.

Nitrobenzene is an aromatic nitro compound and the simplest of the nitrobenzenes, with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents.

Ethylbenzene is an organic compound with the formula C6H5CH2CH3. It is a highly flammable, colorless liquid with an odor similar to that of gasoline. This monocyclic aromatic hydrocarbon is important in the petrochemical industry as a reaction intermediate in the production of styrene, the precursor to polystyrene, a common plastic material. In 2012, more than 99% of ethylbenzene produced was consumed in the production of styrene.

p-Xylene (para-xylene) is an aromatic hydrocarbon. It is one of the three isomers of dimethylbenzene known collectively as xylenes. The p- stands for para-, indicating that the two methyl groups in p-xylene occupy the diametrically opposite substituent positions 1 and 4. It is in the positions of the two methyl groups, their arene substitution pattern, that it differs from the other isomers, o-xylene and m-xylene. All have the same chemical formula C6H4(CH3)2. All xylene isomers are colorless and highly flammable. The odor threshold of p-xylene is 0.62 parts per million (ppm).

Maleic anhydride is an organic compound with the formula C2H2(CO)2O. It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and polymers.

Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.

Diethylenetriamine (abbreviated Dien or DETA) and also known as 2,2’-Iminodi(ethylamine)) is an organic compound with the formula HN(CH2CH2NH2)2. This colourless hygroscopic liquid is soluble in water and polar organic solvents, but not simple hydrocarbons. Diethylenetriamine is structural analogue of diethylene glycol. Its chemical properties resemble those for ethylene diamine, and it has similar uses. It is a weak base and its aqueous solution is alkaline. DETA is a byproduct of the production of ethylenediamine from ethylene dichloride.

Diethanolamine, often abbreviated as DEA or DEOA, is an organic compound with the formula HN(CH2CH2OH)2. Pure diethanolamine is a white solid at room temperature, but its tendencies to absorb water and to supercool often results in it being found in a colorless, viscous liquid state. Diethanolamine is polyfunctional, being a secondary amine and a diol. Like other organic amines, diethanolamine acts as a weak base. Reflecting the hydrophilic character of the secondary amine and hydroxyl groups, DEA is soluble in water. Amides prepared from DEA are often also hydrophilic. In 2013, the chemical was classified by the International Agency for Research on Cancer as "possibly carcinogenic to humans" (Group 2B).

sec-Butyl acetate, or s-butyl acetate, is an ester commonly used as a solvent in lacquers and enamels, where it is used in the production of acyclic polymers, vinyl resins, and nitrocellulose. It is a clear flammable liquid with a sweet smell.
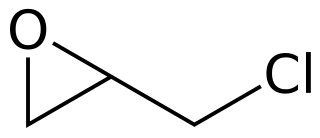
Epichlorohydrin is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organic solvents. It is a chiral molecule generally existing as a racemic mixture of right-handed and left-handed enantiomers. Epichlorohydrin is a highly reactive electrophilic compound and is used in the production of glycerol, plastics, epoxy glues and resins, epoxy diluents and elastomers.
Perchloryl fluoride is a reactive gas with the chemical formula ClO
3F. It has a characteristic sweet odor that resembles gasoline and kerosene. It is toxic and is a powerful oxidizing and fluorinating agent. It is the acid fluoride of perchloric acid.

Glycidol is an organic compound with the formula HOCH2CHOCH2. The molecule contains both epoxide and alcohol functional groups. Being simple to make and bifunctional, it has a variety of industrial uses. The compound is a colorless, slightly viscous liquid that is slightly unstable and is not often encountered in pure form.
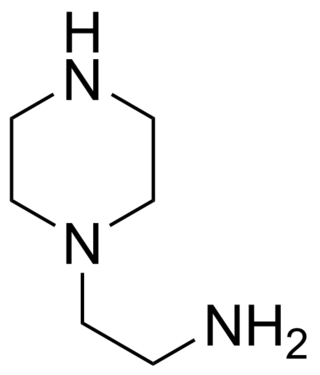
Aminoethylpiperazine (AEP) is a derivative of piperazine. This ethyleneamine contains three nitrogen atoms; one primary, one secondary and one tertiary. It is a corrosive organic liquid and can cause second or third degree burns. Aminoethylpiperazine can also cause pulmonary edema as a result of inhalation. It is REACH and TSCA registered.
4,4′-Methylenedianiline (MDA) is an organic compound with the formula CH2(C6H4NH2)2. It is a colorless solid, although commercial samples can appear yellow or brown. It is produced on an industrial scale, mainly as a precursor to polyurethanes.

Diepoxybutane is an epoxide which is a colorless liquid at room temperature. It is therefore highly reactive, more than other ethers. An epoxide is a cyclic ether that contains a three atom ring that comes close to an equilateral triangle. The primary structure of an epoxide contains two carbon atoms and a hydrocarbon attached to an oxygen atom. It polymerizes in the presence of catalysts or when heated. It’s hydrophilic, very flammable and easily ignited by heat or sparks.
Butyl acrylate is an organic compound with the formula C4H9O2CCH=CH2. A colorless liquid, it is the butyl ester of acrylic acid. It is used commercially on a large scale as a precursor to poly(butyl acrylate). Especially as copolymers, such materials are used in paints, sealants, coatings, adhesives, fuel, textiles, plastics, and caulk.
n-Butyl glycidyl ether is an industrial chemical used in adhesives, sealants, and as a paint or coating additive. It is principally used to reduce the viscosity of epoxy resin systems.

Castor oil glycidyl ether is a liquid organic chemical in the glycidyl ether family. It is sometimes called castor oil triglycidyl ether. It has the theoretical formula C66H116O12. There are two CAS numbers in use, 14228-73-0 and 74398-71-3. The IUPAC name is 2,3-bis[[(E)-12-(oxiran-2-ylmethoxy)octadec-9-enoyl]oxy]propyl (E)-12-(oxiran-2-ylmethoxy)octadec-9-enoate. A key use is acting as a modifier for epoxy resins as a reactive diluent that adds flexibility and improved mechanical properties.
















