Related Research Articles
A transmembrane domain (TMD) is a membrane-spanning protein domain. TMDs may consist of one or several alpha-helices or a transmembrane beta barrel. Because the interior of the lipid bilayer is hydrophobic, the amino acid residues in TMDs are often hydrophobic, although proteins such as membrane pumps and ion channels can contain polar residues. TMDs vary greatly in size and hydrophobicity; they may adopt organelle-specific properties.

Post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes translating mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signalling, as for example when prohormones are converted to hormones.

Peripheral membrane proteins, or extrinsic membrane proteins, are membrane proteins that adhere only temporarily to the biological membrane with which they are associated. These proteins attach to integral membrane proteins, or penetrate the peripheral regions of the lipid bilayer. The regulatory protein subunits of many ion channels and transmembrane receptors, for example, may be defined as peripheral membrane proteins. In contrast to integral membrane proteins, peripheral membrane proteins tend to collect in the water-soluble component, or fraction, of all the proteins extracted during a protein purification procedure. Proteins with GPI anchors are an exception to this rule and can have purification properties similar to those of integral membrane proteins.
Matrix metalloproteinases (MMPs), also known as matrix metallopeptidases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other family members are adamalysins, serralysins, and astacins. The MMPs belong to a larger family of proteases known as the metzincin superfamily.

DD-transpeptidase is a bacterial enzyme that catalyzes the transfer of the R-L-αα-D-alanyl moiety of R-L-αα-D-alanyl-D-alanine carbonyl donors to the γ-OH of their active-site serine and from this to a final acceptor. It is involved in bacterial cell wall biosynthesis, namely, the transpeptidation that crosslinks the peptide side chains of peptidoglycan strands.
PEP group translocation, also known as the phosphotransferase system or PTS, is a distinct method used by bacteria for sugar uptake where the source of energy is from phosphoenolpyruvate (PEP). It is known to be a multicomponent system that always involves enzymes of the plasma membrane and those in the cytoplasm.
Hemagglutinin esterase (HEs) is a glycoprotein that certain enveloped viruses possess and use as an invading mechanism. HEs helps in the attachment and destruction of certain sialic acid receptors that are found on the host cell surface. Viruses that possess HEs include influenza C virus, toroviruses, and coronaviruses of the subgenus Embecovirus. HEs is a dimer transmembrane protein consisting of two monomers, each monomer is made of three domains. The three domains are: membrane fusion, esterase, and receptor binding domains.

A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes. An acid-base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to release the product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine or even selenocysteine. The 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence.
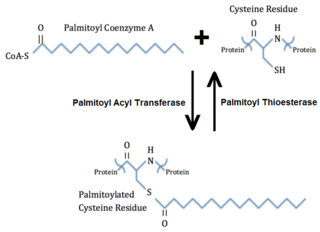
Palmitoylation is the covalent attachment of fatty acids, such as palmitic acid, to cysteine (S-palmitoylation) and less frequently to serine and threonine (O-palmitoylation) residues of proteins, which are typically membrane proteins. The precise function of palmitoylation depends on the particular protein being considered. Palmitoylation enhances the hydrophobicity of proteins and contributes to their membrane association. Palmitoylation also appears to play a significant role in subcellular trafficking of proteins between membrane compartments, as well as in modulating protein–protein interactions. In contrast to prenylation and myristoylation, palmitoylation is usually reversible (because the bond between palmitic acid and protein is often a thioester bond). The reverse reaction in mammalian cells is catalyzed by acyl-protein thioesterases (APTs) in the cytosol and palmitoyl protein thioesterases in lysosomes. Because palmitoylation is a dynamic, post-translational process, it is believed to be employed by the cell to alter the subcellular localization, protein–protein interactions, or binding capacities of a protein.

Carnitine palmitoyltransferase I (CPT1) also known as carnitine acyltransferase I, CPTI, CAT1, CoA:carnitine acyl transferase (CCAT), or palmitoylCoA transferase I, is a mitochondrial enzyme responsible for the formation of acyl carnitines by catalyzing the transfer of the acyl group of a long-chain fatty acyl-CoA from coenzyme A to l-carnitine. The product is often Palmitoylcarnitine, but other fatty acids may also be substrates. It is part of a family of enzymes called carnitine acyltransferases. This "preparation" allows for subsequent movement of the acyl carnitine from the cytosol into the intermembrane space of mitochondria.

Tyrosylprotein sulfotransferase is an enzyme that catalyzes tyrosine sulfation.

Histidine kinases (HK) are multifunctional, and in non-animal kingdoms, typically transmembrane, proteins of the transferase class of enzymes that play a role in signal transduction across the cellular membrane. The vast majority of HKs are homodimers that exhibit autokinase, phosphotransfer, and phosphatase activity. HKs can act as cellular receptors for signaling molecules in a way analogous to tyrosine kinase receptors (RTK). Multifunctional receptor molecules such as HKs and RTKs typically have portions on the outside of the cell that bind to hormone- or growth factor-like molecules, portions that span the cell membrane, and portions within the cell that contain the enzymatic activity. In addition to kinase activity, the intracellular domains typically have regions that bind to a secondary effector molecule or complex of molecules that further propagate signal transduction within the cell. Distinct from other classes of protein kinases, HKs are usually parts of a two-component signal transduction mechanisms in which HK transfers a phosphate group from ATP to a histidine residue within the kinase, and then to an aspartate residue on the receiver domain of a response regulator protein. More recently, the widespread existence of protein histidine phosphorylation distinct from that of two-component histidine kinases has been recognised in human cells. In marked contrast to Ser, Thr and Tyr phosphorylation, the analysis of phosphorylated Histidine using standard biochemical and mass spectrometric approaches is much more challenging, and special procedures and separation techniques are required for their preservation alongside classical Ser, Thr and Tyr phosphorylation on proteins isolated from human cells.

Sterol O-acyltransferase 1, also known as SOAT1, is an enzyme that in humans is encoded by the SOAT1 gene.

Lysophospholipid acyltransferase 5 is an enzyme that in humans is encoded by the LPCAT3 gene. It is homologous to other membrane-bound O-acyltransferases.
In molecular biology the DHHC domain is a protein domain that acts as an enzyme, which adds a palmitoyl chemical group to proteins in order to anchor them to cell membranes. The DHHC domain was discovered in 1999 and named after a conserved sequence motif found in its protein sequence. Roth and colleagues showed that the yeast Akr1p protein could palmitoylate Yck2p in vitro and inferred that the DHHC domain defined a large family of palmitoyltransferases. In mammals twenty three members of this family have been identified and their substrate specificities investigated. Some members of the family such as ZDHHC3 and ZDHHC7 enhance palmitoylation of proteins such as PSD-95, SNAP-25, GAP43, Gαs. Others such as ZDHHC9 showed specificity only toward the H-Ras protein. However, a recent study questions the involvement of classical enzyme-substrate recognition and specificity in the palmitoylation reaction. Several members of the family have been implicated in human diseases.

2-acyl-sn-glycero-3-phosphocholines are a class of phospholipids that are intermediates in the metabolism of lipids. Because they result from the hydrolysis of an acyl group from the sn-1 position of phosphatidylcholine, they are also called 1-lysophosphatidylcholine. The synthesis of phosphatidylcholines with specific fatty acids occurs through the synthesis of 1-lysoPC. The formation of various other lipids generates 1-lysoPC as a by-product.

Lysophospholipid acyltransferase 7 also known as membrane-bound O-acyltransferase domain-containing protein 7 (MBOAT7) is an enzyme that in humans is encoded by the MBOAT7 gene. It is homologous to other membrane-bound O-acyltransferases.

Ghrelin O-acyltransferase also known as membrane bound O-acyltransferase domain containing 4 is an enzyme that in humans is encoded by the MBOAT4 gene. It is homologous to other membrane-bound O-acyltransferases. It is a polytopic membrane protein what takes part in lipid signaling reactions. It is the only known enzyme that catalyzes the acylation of ghrelin through the transfer of n-octanoic acid to ghrelin Ser3. Ghrelin O-acyltransferase function is essential in regulation of appetite and the release of growth hormone. Ghrelin O-acyltransferase is a target for scientific research due to promising applications in the treatment of diabetes, eating disorders, and metabolic diseases.
A proteolipid is a protein covalently linked to lipid molecules, which can be fatty acids, isoprenoids or sterols. The process of such a linkage is known as protein lipidation, and falls into the wider category of acylation and post-translational modification. Proteolipids are abundant in brain tissue, and are also present in many other animal and plant tissues. They include ghrelin, a peptide hormone associated with feeding. Many proteolipids are composed of proteins covalenently bound to fatty acid chains, often granting them an interface for interacting with biological membranes. They are not to be confused with lipoproteins, a kind of spherical assembly made up of many molecules of lipids and some apolipoproteins.
References
- ↑ Hofmann K (March 2000). "A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling". Trends Biochem. Sci. 25 (3): 111–2. doi:10.1016/s0968-0004(99)01539-x. PMID 10694878.
- ↑ Chang, C.C.Y., Sun, J. & Chang, TY. (2011). "Membrane-bound O-acyltransferases (MBOATs)". Front. Biol. 6 (3): 177. doi:10.1007/s11515-011-1149-z. S2CID 41626991.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Wang P, Wang Z, Dou Y, Zhang X, Wang M, Tian X (2013). "Genome-wide identification and analysis of membrane-bound O-acyltransferase (MBOAT) gene family in plants". Planta. 238 (5): 907–22. Bibcode:2013Plant.238..907W. doi:10.1007/s00425-013-1939-4. PMID 23928653. S2CID 1328304.
- ↑ Ma, D; Wang, Z; Merrikh, CN; Lang, KS; Lu, P; Li, X; Merrikh, H; Rao, Z; Xu, W (October 2018). "Crystal structure of a membrane-bound O-acyltransferase". Nature. 562 (7726): 286–290. Bibcode:2018Natur.562..286M. doi:10.1038/s41586-018-0568-2. PMC 6529733 . PMID 30283133.
- ↑ Campaña, Maria B.; Irudayanathan, Flaviyan Jerome; Davis, Tasha R.; McGovern-Gooch, Kayleigh R.; Loftus, Rosemary; Ashkar, Mohammad; Escoffery, Najae; Navarro, Melissa; Sieburg, Michelle A.; Nangia, Shikha; Hougland, James L. (27 September 2019). "The ghrelin O-acyltransferase structure reveals a catalytic channel for transmembrane hormone acylation". The Journal of Biological Chemistry. 294 (39): 14166–14174. doi: 10.1074/jbc.AC119.009749 . ISSN 1083-351X. PMC 6768652 . PMID 31413115.