
PiHKAL: A Chemical Love Story is a book by Dr. Alexander Shulgin and Ann Shulgin, published in 1991. The subject of the work is psychoactive phenethylamine chemical derivatives, notably those that act as psychedelics and/or empathogen-entactogens. The main title, PiHKAL, is an acronym that stands for "Phenethylamines I Have Known and Loved".

2,5-Dimethoxy-4-methylamphetamine is a psychedelic and a substituted amphetamine. It was first synthesized by Alexander Shulgin, and later reported in his book PiHKAL: A Chemical Love Story. DOM is classified as a Schedule I substance in the United States, and is similarly controlled in other parts of the world. Internationally, it is a Schedule I drug under the Convention on Psychotropic Substances. It is generally taken orally.

Escaline (3,5-methoxy-4-ethoxyphenethylamine) is a psychedelic drug and entheogen of the phenethylamine class of compounds. Escaline was first synthesized and reported in the scientific literature by Benington, et al., in 1954, but was later re-examined in the laboratory of David E. Nichols, who prepared a series of mescaline analogues that included escaline, proscaline, and isoproscaline. The effects of this and related mescaline analogues in humans were first described by Alexander Shulgin. In his book PiHKAL , Shulgin lists the dosage range as 40 to 60 mg, consumed orally. The duration of action was stated to be 8–12 hours.
Trimethoxyamphetamines (TMAs) are a family of isomeric psychedelic hallucinogenic drugs. There exist six different TMAs that differ only in the position of the three methoxy groups: TMA, TMA-2, TMA-3, TMA-4, TMA-5, and TMA-6. The TMAs are analogs of the phenethylamine cactus alkaloid mescaline. The TMAs are substituted amphetamines, however, their mechanism of action is more complex than that of the unsubstituted compound amphetamine, probably involving agonist activity on serotonin receptors such as the 5HT2A receptor in addition to the generalised dopamine receptor agonism typical of most amphetamines. This action on serotonergic receptors likely underlie the psychedelic effects of these compounds. It is reported that some TMAs elicit a range of emotions ranging from sadness to empathy and euphoria. TMA was first synthesized by Hey, in 1947. Synthesis data as well as human activity data has been published in the book PiHKAL.

MMDA is a psychedelic and entactogen drug of the amphetamine class. It is an analogue of lophophine, MDA, and MDMA.

3,4-Methylenedioxy-N-methoxyamphetamine is a lesser-known psychedelic drug and a substituted amphetamine. It is also the N-methoxy analogue of MDA. MDMEO was first synthesized by Alexander Shulgin. In his book PiHKAL , the minimum dosage is listed as 180 mg. MDMEO may be found as white crystals. It produces few to no effects. Very little data exists about the pharmacological properties, metabolism, and toxicity of MDMEO.
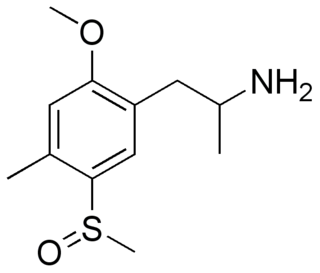
TOMSO (2-methoxy]]-4-methyl-5-methylsulfinylamphetamine) is a lesser-known psychedelic drug and a substituted amphetamine. TOMSO was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage range is listed as 100–150 mg, and the duration listed as 10–16 hours. TOMSO is inactive on its own; it is activated with the consumption of alcohol. It produces intense time distortion and a threshold. Very little data exists about the pharmacological properties, metabolism, and toxicity of TOMSO.

Ψ-DOM, or 2,6-dimethoxy-4-methylamphetamine, is a hallucinogenic, psychedelic drug and a structural isomer of the better-known hallucinogen DOM. Ψ-DOM was first reported by Alexander Shulgin in his book PiHKAL.

BOB (4-bromo-2,5,beta-trimethoxyphenethylamine) is a lesser-known psychedelic drug. It is the beta-methoxy analog of 2C-B. BOB was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage range is listed as 10–20 mg, and the duration listed as 10–20 hours. BOB produces an altered state of consciousness, tinnitus, a pleasant tingling throughout the body, and a sense of awareness. Very little data exists about the pharmacological properties, metabolism, and toxicity of BOB.

BOD (4-methyl-2,5,β-trimethoxyphenethylamine) is a lesser-known psychedelic drug. It is the beta-methoxy analog of 2C-D. BOD was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage range is listed as 15–25 mg, and the duration listed as 8–16 hours. BOD produces strongly distorted open-eye visuals, and some closed-eye visuals. It also has an entheogenic effect and produces humor. Very little data exists about the pharmacological properties, metabolism, and toxicity of BOD.

BOM (3,4,5,beta-tetramethoxyphenethylamine) is a lesser-known psychedelic drug. It is the beta-methoxy derivative of mescaline. BOM was first synthesized by Alexander Shulgin. In his book PiHKAL, the minimum dosage is listed as 200 mg, and the duration unknown. BOM produces few to no effects. Very little data exists about its pharmacological properties, metabolism, and toxicity.
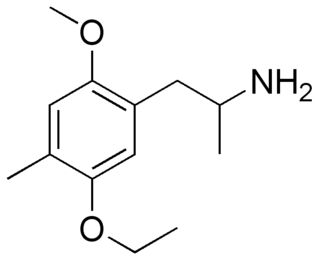
IRIS (2-methoxy-5-ethoxy-4-methylamphetamine) is a lesser-known psychedelic drug and a substituted amphetamine. It is also the 5-ethoxy analog of DOM. IRIS was first synthesized by Alexander Shulgin. In his book PiHKAL, the minimum dosage is listed as 9 mg, and the duration unknown. IRIS produces few to no effects. Very little data exists about the pharmacological properties, metabolism, and toxicity of IRIS.

EEM (2,4-diethoxy-5-methoxyamphetamine) is a lesser-known substituted amphetamine. It is a diethoxy-methoxy analog of trimethoxyamphetamine (TMA-2). EEM was first synthesized by Alexander Shulgin. In his book PiHKAL, both the dosage and duration are unknown. EEM produces few to no effects. Very little data exists about the pharmacological properties, metabolism, and toxicity of EEM.

EME (2,5-diethoxy-4-methoxyamphetamine) is a lesser-known psychedelic drug. It is a diethoxy-methoxy analog of TMA-2. EME was first synthesized by Alexander Shulgin. In his book PiHKAL, both the dosage and duration are unknown. EME produces few to no effects. Very little data exists about the pharmacological properties, metabolism, and toxicity of EME.

F-2, or 6-(2-aminopropyl)-5-methoxy-2-methyl-2,3-dihydrobenzofuran, is a lesser-known psychedelic drug. F-2 was first synthesized by Alexander Shulgin. In his book PiHKAL, the minimum dosage is listed as 15 mg, and the duration unknown. F-2 produces few to no effects at this dose in humans. Animal studies showed it to substitute for the psychedelic drug DOM, but with less than one tenth the potency.

MEPEA, or 3-methoxy-4-ethoxyphenethylamine, is a lesser-known psychedelic drug. MEPEA was first synthesized by Alexander Shulgin. In his book PiHKAL , the minimum dosage is listed as 300 mg, and the duration unknown. MEPEA produces a light lifting feeling and a +1 on the Shulgin Rating Scale. Very little data exists about the pharmacological properties, metabolism, and toxicity of MEPEA.
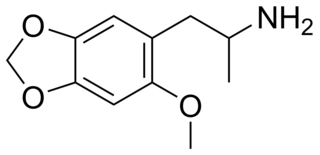
MMDA-2 (2-methoxy-4,5-methylenedioxyamphetamine) is a psychedelic drug of the amphetamine class. It is closely related to MMDA and MDA.

N-Methyl-2-methoxy-4,5-methylenedioxyamphetamine is a psychedelic drug of the amphetamine class. It is the N-methylated derivative of MMDA-2, and it is also an analog of MDMA and 6-methyl-MDA.

MEDA (3-methoxy-4,5-ethylenedioxyamphetamine) is a lesser-known psychedelic drug. MEDA was first synthesized by Alexander Shulgin. In his book PiHKAL, the minimum dosage is listed as 200 mg, and the duration unknown. MEDA produces few to no effects. Very little data exists about the pharmacological properties, metabolism, and toxicity of MEDA.

2CD-5EtO is a homologue of the psychedelic phenethylamine 2C-D, where the 5-methoxy group of 2C-D has been lengthened to an ethoxy group. 2CD-5EtO is a representative example of the so-called "tweetio" compounds discovered by Alexander Shulgin and briefly mentioned in his book PiHKAL. They are homologues of the 2C family of drugs, where either one or both of the methoxy groups at the 2,5-positions of the aromatic ring have been replaced by ethoxy. The term tweetio was derived phonetically from the sound of the "2-ETO" derivatives.



















