Proteasomes are protein complexes which degrade ubiquitin-tagged proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases.

A calpain is a protein belonging to the family of calcium-dependent, non-lysosomal cysteine proteases expressed ubiquitously in mammals and many other organisms. Calpains constitute the C2 family of protease clan CA in the MEROPS database. The calpain proteolytic system includes the calpain proteases, the small regulatory subunit CAPNS1, also known as CAPN4, and the endogenous calpain-specific inhibitor, calpastatin.

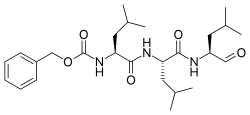
Proteasome inhibitors are drugs that block the action of proteasomes, cellular complexes that break down proteins. They are being studied in the treatment of cancer; three are approved for use in treating multiple myeloma.
The IκB kinase is an enzyme complex that is involved in propagating the cellular response to inflammation, specifically the regulation of lymphocytes.

26S protease regulatory subunit 6A, also known as 26S proteasome AAA-ATPase subunit Rpt5, is an enzyme that in humans is encoded by the PSMC3 gene. This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.
Proteasome subunit beta type-4 also known as 20S proteasome subunit beta-7 is a protein that in humans is encoded by the PSMB4 gene.

Proteasome subunit beta type-10 as known as 20S proteasome subunit beta-2i is a protein that in humans is encoded by the PSMB10 gene.
26S protease regulatory subunit 8, also known as 26S proteasome AAA-ATPase subunit Rpt6, is an enzyme that in humans is encoded by the PSMC5 gene. This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.

26S protease regulatory subunit 7, also known as 26S proteasome AAA-ATPase subunit Rpt1, is an enzyme that in humans is encoded by the PSMC2 gene This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex. Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.
Proteasome subunit beta type-5 also known as 20S proteasome subunit beta-5 is a protein that in humans is encoded by the PSMB5 gene. This protein is one of the 17 essential subunits that contributes to the complete assembly of 20S proteasome complex. In particular, proteasome subunit beta type-5, along with other beta subunits, assemble into two heptameric rings and subsequently a proteolytic chamber for substrate degradation. This protein contains "chymotrypsin-like" activity and is capable of cleaving after large hydrophobic residues of peptide. The eukaryotic proteasome recognized degradable proteins, including damaged proteins for protein quality control purpose or key regulatory protein components for dynamic biological processes. An essential function of a modified proteasome, the immunoproteasome, is the processing of class I MHC peptides.
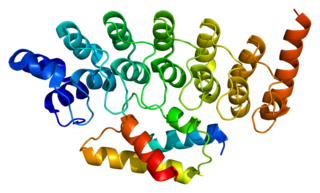
26S protease regulatory subunit 6B, also known as 26S proteasome AAA-ATPase subunit Rpt3, is an enzyme that in humans is encoded by the PSMC4 gene. This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.

26S proteasome non-ATPase regulatory subunit 7, also known as 26S proteasome non-ATPase subunit Rpn8, is an enzyme that in humans is encoded by the PSMD7 gene.

Proteasome activator complex subunit 2 is a protein that in humans is encoded by the PSME2 gene.

Proteasome inhibitor PI31 subunit is a protein that in humans is encoded by the PSMF1 gene.

26S protease regulatory subunit S10B, also known as 26S proteasome AAA-ATPase subunit Rpt4, is an enzyme that in humans is encoded by the PSMC6 gene. This protein is one of the 19 essential subunits of a complete assembled 19S proteasome complex Six 26S proteasome AAA-ATPase subunits together with four non-ATPase subunits form the base sub complex of 19S regulatory particle for proteasome complex.

26S proteasome non-ATPase regulatory subunit 8 is an enzyme that in humans is encoded by the PSMD8 gene.

26S proteasome non-ATPase regulatory subunit 3 is an enzyme that in humans is encoded by the PSMD3 gene.
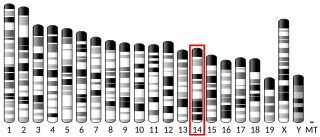
26S proteasome non-ATPase regulatory subunit 6 is an enzyme that in humans is encoded by the PSMD6 gene.
Proteostasis is the dynamic regulation of a balanced, functional proteome. The proteostasis network includes competing and integrated biological pathways within cells that control the biogenesis, folding, trafficking, and degradation of proteins present within and outside the cell. Loss of proteostasis is central to understanding the cause of diseases associated with excessive protein misfolding and degradation leading to loss-of-function phenotypes, as well as aggregation-associated degenerative disorders. Therapeutic restoration of proteostasis may treat or resolve these pathologies.
Alfred Lewis Goldberg was an American cell biologist-biochemist and professor at Harvard University. His major discoveries have concerned the mechanisms and physiological importance of protein degradation in cells. Of wide impact have been his lab's demonstration that all cells contain a pathway for selectively eliminating misfolded proteins, his discoveries about the role of proteasomes in this process and of the enzyme systems catalyzing protein breakdown in bacteria, his elucidating the mechanisms for muscle atrophy and the role of proteasomes in antigen presentation to the immune system, and his introduction of proteasome inhibitors now widely used as research tools and in the treatment of blood cancers.