Saponins are bitter-tasting usually toxic plant-derived secondary metabolites, being organic chemicals, that have a foamy quality when agitated in water and a high molecular weight. They are present in a wide range of plant species throughout the bark, leaves, stems, roots and flowers but found particularly in soapwort, a flowering plant, the soapbark tree, common corn-cockle, baby’s breath and soybeans. They are used in soaps, medicines, fire extinguishers, as dietary supplements, for synthesis of steroids, and in carbonated beverages. Saponins are both water and fat soluble, which gives them their useful soap properties. Some examples of these chemicals are glycyrrhizin and quillaia, a bark extract used in beverages.
Okadaic acid, C44H68O13, is a toxin produced by several species of dinoflagellates, and is known to accumulate in both marine sponges and shellfish. One of the primary causes of diarrhetic shellfish poisoning, okadaic acid is a potent inhibitor of specific protein phosphatases and is known to have a variety of negative effects on cells. A polyketide, polyether derivative of a C38 fatty acid, okadaic acid and other members of its family have shined light upon many biological processes both with respect to dinoflagellete polyketide synthesis as well as the role of protein phosphatases in cell growth.

2-Imidazoline (Preferred IUPAC name: 4,5-dihydro-1H-imidazole) is one of three isomers of the nitrogen-containing heterocycle imidazoline, with the formula C3H6N2. The 2-imidazolines are the most common imidazolines commercially, as the ring exists in some natural products and some pharmaceuticals. They also have been examined in the context of organic synthesis, coordination chemistry, and homogeneous catalysis.
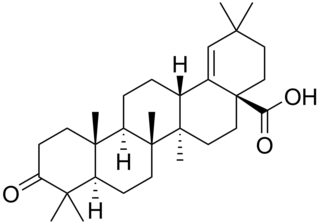
Moronic acid is a natural triterpene. Moronic acid can be extracted from Rhus javanica, a sumac plant traditionally believed to hold medicinal applications. The molecule has also been extracted from mistletoe.

Triterpenes are a class of terpenes composed of six isoprene units with the molecular formula C30H48; they may also be thought of as consisting of three terpene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids.
A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR-. Many depsipeptides have both peptide and ester linkages. Elimination of the N–H group in a peptide structure results in a decrease of H-bonding capability, which is responsible for secondary structure and folding patterns of peptides, thus inducing structural deformation of the helix and β-sheet structures. Because of decreased resonance delocalization in esters relative to amides, depsipeptides have lower rotational barriers for cis-trans isomerization and therefore they have more flexible structures than their native analogs. They are mainly found in marine and microbial natural products.

Combretastatin is a dihydrostilbenoid found in Combretum afrum.

5-Bromo-DMT (5-bromo-N,N-dimethyltryptamine) is a psychedelic brominated indole alkaloid found in the sponges Smenospongia aurea and Smenospongia echina, as well as in Verongula rigida alongside 5,6-Dibromo-DMT and seven other alkaloids. It is the 5-bromo derivative of DMT, a psychedelic found in many plants and animals.
Iodolactonization is an organic reaction that forms a ring by the addition of an oxygen and iodine across a carbon-carbon double bond. It is an intramolecular variant of the halohydrin synthesis reaction. The reaction was first reported by M. J. Bougalt in 1904 and has since become one of the most effective ways to synthesize lactones. Strengths of the reaction include the mild conditions and incorporation of the versatile iodine atom into the product.

Palau'amine is a toxic chlorinated alkaloid compound synthesized naturally by certain species of sea sponges. The name of the molecule derives from the island nation of Palau, near where the first sponge species discovered to produce it, Stylotella agminata, is found. It has since been isolated in other sponges, including Stylissa massa.

Capnellene is a naturally occurring tricyclic hydrocarbon derived from Capnella imbricata, a species of soft coral found in Indonesia. Since the 1970s, capnellene has been targeted for synthesis by numerous investigators due to its stereochemistry, functionality, and the interesting geometry of the carbon skeleton. Many alcohol derivatives of capnellene have demonstrated potential as a chemotherapeutic agent with antibacterial, anti-inflammatory and anti-tumor properties.

Rhabdastrella globostellata, also known as the yellow pot sponge, is a marine sponge of the order Astrophorida. It is native to many regions of the Indian Ocean including the shores of Madagascar, the Seychelles, and Australia as well as the Malayan Peninsula and Singapore. It was first described by Henry J. Carter as Stelleta globostellata in 1883, named after the globostellate shape of its spicules.
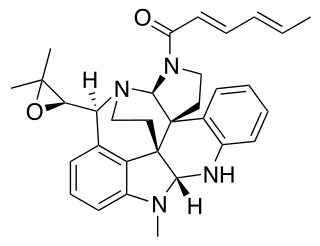
Communesin B is a cytotoxic chemical compound isolated from Penicillium strains found on the marine alga Ulva intestinalis. It exhibits cytotoxicity in vitro against human lung carcinoma, prostate carcinoma, colorectal carcinoma, cervical adenocarcinoma, and breast adenocarcinoma cell lines.

Oroidin is a bromopyrrole alkaloid, originally isolated from marine sponges in the genus Agelas. It appears to have a wide range of biological activities, which makes Oroidin a potential drug candidate for various diseases. It also serves as chemical defense in marine sponges.
Isotuberculosinol, also called nosyberkol or edaxadiene is a diterpene molecule produced by the bacterium Mycobacterium tuberculosis, the causative agent of TB, which aids in its pathogenesis. Isotuberculosinol functions by preventing maturation of the host-cell phagosome in which the bacterium lives. Maturation of the phagosome would enable it to kill the bacterium. Mutations in genes involved in the biosynthetic pathway of nosyberkol result in normal development of the phagosome and reduction of mycobacterial infection. These biosynthetic genes include isotuberculosinol synthase.
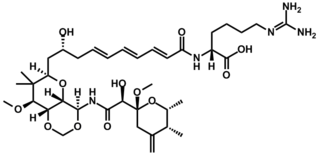
Onnamide A is a bioactive natural product found in Theonella swinhoei, a species of marine sponge whose genus is well known for yielding a diverse set of biologically active natural products, including the swinholides and polytheonamides. It bears structural similarities to the pederins, a family of compounds known to inhibit protein synthesis in eukaryotic cells. Onnamide A and its analogues have attracted academic interest due to their cytotoxicity and potential for combating the growth and proliferation of cancer cells.
Kalkitoxin, a toxin derived from the cyanobacterium Lyngbya majuscula, induces NMDA receptor mediated neuronal necrosis, blocks voltage-dependent sodium channels, and induces cellular hypoxia by inhibiting the electron transport chain (ETC) complex 1.

Swinholides are dimeric 42 carbon-ring polyketides that exhibit a 2-fold axis of symmetry. Found mostly in the marine sponge Theonella, swinholides encompass cytotoxic and antifungal activities via disruption of the actin skeleton. Swinholides were first described in 1985 and the structure and stereochemistry were updated in 1989 and 1990, respectively. Thirteen swinholides have been described in the literature, including close structural compounds such as misakinolides/bistheonellides, ankaraholides, and hurgholide A It is suspected that symbiotic microbes that inhabit the sponges rather than the sponges themselves produce swinholides since the highest concentration of swinholides are found in the unicellular bacterial fraction of sponges and not in the sponge fraction or cyanobacteria fraction that also inhabit the sponges.
Dysidazirine is a organic compound with formula C19H33NO2. It was discovered as a natural product in 1988 in the marine sponge Dysidea fragilis. Chemically, it is a 2H-azirine derivative.
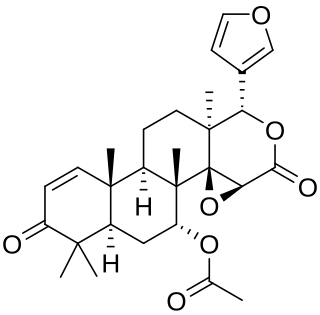
Gedunin is a pentacyclic triterpenoid with the molecular formula C28H34 O7. It is most notably found in Azadirachta indica, but is a constituent of several other plants. Gedunin shows therapeutic potential in the treatment of leukemia, and Parkinson's disease.













