
Jaroslav Heyrovský was a Czech chemist and inventor. Heyrovský was the inventor of the polarographic method, father of the electroanalytical method, and recipient of the Nobel Prize in 1959 for his invention and development of the polarographic methods of analysis. His main field of work was polarography.

In electrochemistry, cyclic voltammetry (CV) is a type of potentiodynamic measurement. In a cyclic voltammetry experiment, the working electrode potential is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles of ramps in potential may be repeated as many times as needed. The current at the working electrode is plotted versus the applied voltage to give the cyclic voltammogram trace. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode.

Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied. The analytical data for a voltammetric experiment comes in the form of a voltammogram which plots the current produced by the analyte versus the potential of the working electrode.
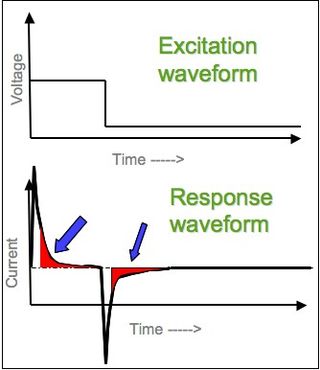
In electrochemistry, chronoamperometry is an analytical technique in which the electric potential of the working electrode is stepped and the resulting current from faradaic processes occurring at the electrode is monitored as a function of time. The functional relationship between current response and time is measured after applying single or double potential step to the working electrode of the electrochemical system. Limited information about the identity of the electrolyzed species can be obtained from the ratio of the peak oxidation current versus the peak reduction current. However, as with all pulsed techniques, chronoamperometry generates high charging currents, which decay exponentially with time as any RC circuit. The Faradaic current - which is due to electron transfer events and is most often the current component of interest - decays as described in the Cottrell equation. In most electrochemical cells, this decay is much slower than the charging decay-cells with no supporting electrolyte are notable exceptions. Most commonly a three-electrode system is used. Since the current is integrated over relatively longer time intervals, chronoamperometry gives a better signal-to-noise ratio in comparison to other amperometric techniques.
Polarography is a type of voltammetry where the working electrode is a dropping mercury electrode (DME) or a static mercury drop electrode (SMDE), which are useful for their wide cathodic ranges and renewable surfaces. It was invented in 1922 by Czech chemist Jaroslav Heyrovský, for which he won the Nobel prize in 1959. The main advantages of mercury as electrode material are as follows: 1) a large voltage window: ca. from +0.2 V to -1.8 V vs reversible hydrogen electrode (RHE). Hg electrode is particularly well-suited for studying electroreduction reactions. 2) very reproducible electrode surface, since mercury is liquid. 3) very easy cleaning of the electrode surface by making a new drop of mercury from a large Hg pool connected by a glass capillary.

Richard Guy Compton FRSC MAE is Professor of Chemistry and Aldrichian Praelector at Oxford University, United Kingdom. He is a Tutorial Fellow of St John’s College, Oxford and has a large research group based at the Physical and Theoretical Chemistry Laboratory at Oxford University. Compton has broad interests in both fundamental and applied electrochemistry and electro-analysis including nano-chemical aspects. He has published more than 1600 papers with more than 44,000 citations, excluding self-cites, as of March 2020; Reuters-Thomson ‘Highly Cited Researcher’ 2014, 2015 and 2016) and 7 books.
Electroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. These methods can be broken down into several categories depending on which aspects of the cell are controlled and which are measured. The four main categories are potentiometry, amperometry, coulometry, and voltammetry.
An ultramicroelectrode (UME) is a working electrode used in a voltammetry. The small size of UME give them large diffusion layers and small overall currents. These features allow UME to achieve useful steady-state conditions and very high scan rates (V/s) with limited distortion. UME were developed independently by Wightman and Fleischmann around 1980. Small current at UME enables electrochemical measurements in low conductive media, where voltage drop associated with high solution resistance makes these experiments difficult for conventional electrodes. Furthermore, small voltage drop at UME leads to a very small voltage distortion at the electrode-solution interface which allows using two-electrode setup in voltammetric experiment instead of conventional three-electrode setup.

Joseph Wang is an American biomedical engineer and inventor. He is a Distinguished Professor, SAIC Endowed Chair, and former Chair of the Department of Nanoengineering at the University of California, San Diego, who specialised in nanomachines, biosensors, nano-bioelectronics, wearable devices, and electrochemistry. He is also the Director of the UCSD Center of Wearable Sensors and co-director of the UCSD Center of Mobile Health Systems and Applications (CMSA).

Allen Joseph Bard is an American chemist. He is the Hackerman-Welch Regents Chair Professor and director of the Center for Electrochemistry at the University of Texas at Austin. Bard is considered a "father of modern electrochemistry" for his innovative work developing the scanning electrochemical microscope, his co-discovery of electrochemiluminescence, his key contributions to photoelectrochemistry of semiconductor electrodes, and co-authoring a seminal textbook.

Martin Fleischmann FRS was a British chemist who worked in electrochemistry. Premature announcement of his cold fusion research with Stanley Pons, regarding excess heat in heavy water, caused a media sensation and elicited skepticism and criticism from many in the scientific community.

Fast-scan cyclic voltammetry (FSCV) is cyclic voltammetry with a very high scan rate (up to 1×106 V·s−1). Application of high scan rate allows rapid acquisition of a voltammogram within several milliseconds and ensures high temporal resolution of this electroanalytical technique. An acquisition rate of 10 Hz is routinely employed.
Debra R. Rolison is a physical chemist at the Naval Research Laboratory, where she is a head of the Advanced Electrochemical Materials section. Rolison's research involves the design, synthesis, and characterization of multi-functional nanostructures and ultra porous materials for rate-critical applications such as catalysis and energy storage. She is the 112th recipient of the William H. Nichols Medal Award.

Royce W. Murray was an American chemist and chemistry professor at the University of North Carolina at Chapel Hill. His research interests were focused on electrochemistry, molecular designs, and sensors. He published over 440 peer-reviewed articles in analytical, physical, inorganic, and materials chemistry, and trained 72 Ph.D students, 16 master’s students, and 58 postdoctoral fellows, 45 of whom have gone on to university faculty positions. He was named a fellow of the American Chemical Society in 2012, and was the inventor on three patents related to surface-modified electrodes.
Shelley D. Minteer is an American academic and chemistry professor at the University of Utah. Minteer field of study focuses on the interface between biocatalysts and enzyme-based electrodes for biofuel cells and sensors.

Samuel Kounaves is an American researcher, academic and author. He is a Professor of Chemistry and adjunct Professor in Earth & Ocean Sciences at Tufts University. He also holds a visiting professorship at Imperial College London since 2014, a Consulting Scientist position at the Technical University of Berlin, and is an affiliate scientist at NASA’s Jet Propulsion Laboratory.

Parastoo ("Parry") Hashemi is an Iranian-British neural engineer at Imperial College London and the University of South Carolina.
B. Jill Venton is a professor of chemistry at University of Virginia, where she serves as the department chair since 2019. Venton's research focuses on developing analytical chemistry methods to enable detection of molecules in the brain.
Janet Gretchen Osteryoung was an American chemist who was the director of the Chemistry Division of the National Science Foundation from 1994 to 2001. Her research furthered the development of electroanalysis and especially that of square wave voltammetry. She was elected a Fellow of the American Association for the Advancement of Science in 1984 and awarded the Garvan–Olin Medal in 1987.

Theodore Kuwana (1931–2022) was a chemist and academic researcher known as the founding father of the field of spectroelectrochemistry.














