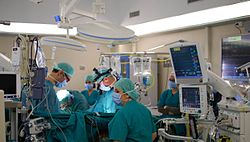

Medical gowns are hospital gowns worn by medical professionals as personal protective equipment (PPE) in order to provide a barrier between patient and professional. Whereas patient gowns are flimsy often with exposed backs and arms, PPE gowns, as seen below in the cardiac surgeon photograph, cover most of the exposed skin surfaces of the professional medics.
Contents
- History
- Types
- Local variants
- United States
- China
- European Union
- Israel
- Criticisms
- See also
- References
In several countries, PPE gowns for use in the COVID-19 pandemic became in appearance more like cleanroom suits as knowledge of the best practices filtered up through the national bureaucracies. For example, the European norm-setting bodies CEN and CENELEC on 30 March 2020 in collaboration with the European Commissioner for the Internal Market made freely-available the relevant standards documents in order "to tackle the severe shortage of protective masks, gloves and other products currently faced by many European countries. Providing free access to the standards will facilitate the work of the many companies wishing to reconvert their production lines in order to manufacture the equipment that is so urgently needed." [2]

