
Turmeric, ,) is a flowering plant in the ginger family Zingiberaceae. It is a perennial, rhizomatous, herbaceous plant native to the Indian subcontinent and Southeast Asia that requires temperatures between 20 and 30 °C and high annual rainfall to thrive. Plants are gathered each year for their rhizomes, some for propagation in the following season and some for consumption.

Kavalactones are a class of lactone compounds found in kava roots and Alpinia zerumbet. Some kavalactones are bioactive.

Curcumin is a bright yellow chemical produced by plants of the Curcuma longa species. It is the principal curcuminoid of turmeric, a member of the ginger family, Zingiberaceae. It is sold as a herbal supplement, cosmetics ingredient, food flavoring, and food coloring.

Deoxycholic acid is a bile acid. Deoxycholic acid is one of the secondary bile acids, which are metabolic byproducts of intestinal bacteria. The two primary bile acids secreted by the liver are cholic acid and chenodeoxycholic acid. Bacteria metabolize chenodeoxycholic acid into the secondary bile acid lithocholic acid, and they metabolize cholic acid into deoxycholic acid. There are additional secondary bile acids, such as ursodeoxycholic acid. Deoxycholic acid is soluble in alcohol and acetic acid. When pure, it exists in a white to off-white crystalline powder form.
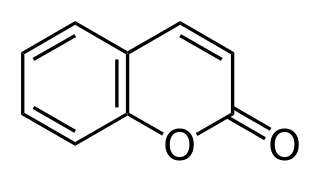
Coumarin or 2H-chromen-2-one is an aromatic organic chemical compound with formula C9H6O2. Its molecule can be described as a benzene molecule with two adjacent hydrogen atoms replaced by an unsaturated lactone ring −(CH)=(CH)−(C=O)−O−, forming a second six-membered heterocycle that shares two carbons with the benzene ring. It belongs to the benzopyrone chemical class and considered as a lactone.

Brilliant blue FCF is a synthetic organic compound used primarily as a blue colorant for processed foods, medications, dietary supplements, and cosmetics. It is classified as a triarylmethane dye and is known under various names, such as FD&C Blue No. 1 or acid blue 9. It is denoted by E number E133 and has a color index of 42090. It has the appearance of a blue powder and is soluble in water and glycerol, with a maximum absorption at about 628 nanometers. It is one of the oldest FDA-approved color additives and is generally considered nontoxic and safe.

Pulegone is a naturally occurring organic compound obtained from the essential oils of a variety of plants such as Nepeta cataria (catnip), Mentha piperita, and pennyroyal. It is classified as a monoterpene.

Hypervitaminosis A refers to the toxic effects of ingesting too much preformed vitamin A. Symptoms arise as a result of altered bone metabolism and altered metabolism of other fat-soluble vitamins. Hypervitaminosis A is believed to have occurred in early humans, and the problem has persisted throughout human history. Toxicity results from ingesting too much preformed vitamin A from foods, supplements, or prescription medications and can be prevented by ingesting no more than the recommended daily amount.

Iproniazid is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class. It is a xenobiotic that was originally designed to treat tuberculosis, but was later most prominently used as an antidepressant drug. However, it was withdrawn from the market because of its hepatotoxicity. The medical use of iproniazid was discontinued in most of the world in the 1960s, but remained in use in France until its discontinuation in 2015.
Toluene toxicity refers to the harmful effects caused by toluene on the body.

Diphenylamine is an organic compound with the formula (C6H5)2NH. The compound is a derivative of aniline, consisting of an amine bound to two phenyl groups. The compound is a colorless solid, but commercial samples are often yellow due to oxidized impurities. Diphenylamine dissolves well in many common organic solvents, and is moderately soluble in water. It is used mainly for its antioxidant properties. Diphenylamine is widely used as an industrial antioxidant, dye mordant and reagent and is also employed in agriculture as a fungicide and antihelmintic.
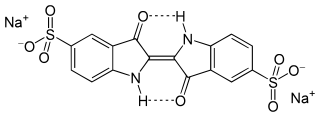
Indigo carmine, or 5,5′-indigodisulfonic acid sodium salt, is an organic salt derived from indigo by aromatic sulfonation, which renders the compound soluble in water. Like indigo, it produces a blue color, and is used in food and other consumables, cosmetics, and as a medical contrast agent and staining agent; it also acts as a pH indicator. It is approved for human consumption in the United States and European Union. It has the E number E132, and is named Blue No. 2 by the US Federal Food, Drug, and Cosmetic Act.

Pyrrolizidine alkaloids (PAs), sometimes referred to as necine bases, are a group of naturally occurring alkaloids based on the structure of pyrrolizidine. Their use dates back centuries and is intertwined with the discovery, understanding, and eventual recognition of their toxicity on humans and animals.

Terminalia chebula, commonly known as black- or chebulic myrobalan, is a species of Terminalia, native to South Asia from Pakistan, India and Nepal east to southwest China (Yunnan), and south to Sri Lanka, Malaysia, and Vietnam.

Protocatechuic acid (PCA) is a dihydroxybenzoic acid, a type of phenolic acid. It is a major metabolite of antioxidant polyphenols found in green tea. It has mixed effects on normal and cancer cells in in vitro and in vivo studies. It is produced commercially from vanillin.

Cornus officinalis, the Japanese cornel or Japanese cornelian cherry, is a species of flowering plant in the dogwood family Cornaceae. Despite its name, it is native to China and Korea as well as Japan. It is not to be confused with C. mas, which is also known as the Cornelian cherry. It is not closely related to the true cherries of the genus Prunus.
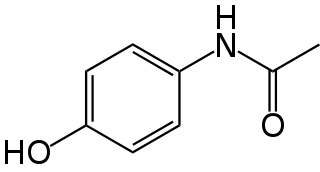
Paracetamol poisoning, also known as acetaminophen poisoning, is caused by excessive use of the medication paracetamol (acetaminophen). Most people have few or non-specific symptoms in the first 24 hours following overdose. These symptoms include feeling tired, abdominal pain, or nausea. This is typically followed by absence of symptoms for a couple of days, after which yellowish skin, blood clotting problems, and confusion occurs as a result of liver failure. Additional complications may include kidney failure, pancreatitis, low blood sugar, and lactic acidosis. If death does not occur, people tend to recover fully over a couple of weeks. Without treatment, death from toxicity occurs 4 to 18 days later.

Diethyl phthalate (DEP) is a phthalate ester. It occurs as a colourless liquid without significant odour but has a bitter, disagreeable taste. It is more dense than water and insoluble in water; hence, it sinks in water.

25B-NBOMe is a derivative of the phenethylamine psychedelic 2C-B, discovered in 2004 by Ralf Heim at the Free University of Berlin. It acts as a potent full agonist for the 5HT2A receptor. Duration of effects lasts about 3–10 hours, although the parent compound is rapidly cleared from the blood when used in the radiolabeled form in tracer doses. Recently, Custodio et al. (2019) evaluated the potential involvement of dysregulated dopaminergic system, neuroadaptation, and brain wave changes which may contribute to the rewarding and reinforcing properties of 25B-NBOMe in rodents.

Amanita sphaerobulbosa, commonly known as the Asian abrupt-bulbed Lepidella, is a species of agaric fungus in the family Amanitaceae. First described by mycologist Tsuguo Hongo in 1969, it is found in Southern Asia.



















